Hyperglycemia enhances function and differentiation of adult rat cardiac fibroblasts
- PMID: 24959995
- PMCID: PMC4883005
- DOI: 10.1139/cjpp-2013-0490
Hyperglycemia enhances function and differentiation of adult rat cardiac fibroblasts
Abstract
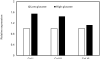
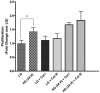
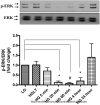
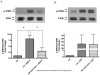
Diabetes is an independent risk factor for cardiovascular disease that can eventually cause cardiomyopathy and heart failure. Cardiac fibroblasts (CF) are the critical mediators of physiological and pathological cardiac remodeling; however, the effects of hyperglycemia on cardiac fibroblast function and differentiation is not well known. Here, we performed a comprehensive investigation on the effects of hyperglycemia on cardiac fibroblasts and show that hyperglycemia enhances cardiac fibroblast function and differentiation. We found that high glucose treatment increased collagen I, III, and VI gene expression in rat adult cardiac fibroblasts. Interestingly, hyperglycemia increased CF migration and proliferation that is augmented by collagen I and III. Surprisingly, we found that short term hyperglycemia transiently inhibited ERK1/2 activation but increased AKT phosphorylation. Finally, high glucose treatment increased spontaneous differentiation of cardiac fibroblasts to myofibroblasts with increasing passage compared with low glucose. Taken together, these findings suggest that hyperglycemia induces cardiac fibrosis by modulating collagen expression, migration, proliferation, and differentiation of cardiac fibroblasts.
Keywords: cardiac fibroblast; collagen; collagène; concentration élevée de glucose; diabetes; diabète; fibroblaste cardiaque; high glucose; migration; myofibroblast; myofibroblastes; proliferation; prolifération.
Figures

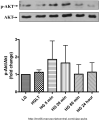
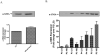
References
-
- Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O, Boccuzzi G. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–388. - PubMed
-
- Asbun J, Manso AM, Villarreal FJ. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: effects of losartan and vitamin E. Am J Physiol Heart Circ Physiol. 2005;288:H227–H234. - PubMed
-
- Asbun J, Villarreal FJ. The Pathogenesis of Myocardial Fibrosis in the Setting of Diabetic Cardiomyopathy. Journal of American College of Cardiology. 2006;47:693–700. - PubMed
-
- Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia. 1978;14:363–370. - PubMed
-
- Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes. 1984;33:271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

