Actions of hydrogen sulfide on sodium transport processes across native distal lung epithelia (Xenopus laevis)
- PMID: 24960042
- PMCID: PMC4069190
- DOI: 10.1371/journal.pone.0100971
Actions of hydrogen sulfide on sodium transport processes across native distal lung epithelia (Xenopus laevis)
Abstract
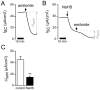
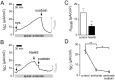
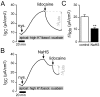
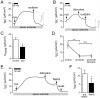
Hydrogen sulfide (H2S) is well known as a highly toxic environmental chemical threat. Prolonged exposure to H2S can lead to the formation of pulmonary edema. However, the mechanisms of how H2S facilitates edema formation are poorly understood. Since edema formation can be enhanced by an impaired clearance of electrolytes and, consequently, fluid across the alveolar epithelium, it was questioned whether H2S may interfere with transepithelial electrolyte absorption. Electrolyte absorption was electrophysiologically measured across native distal lung preparations (Xenopus laevis) in Ussing chambers. The exposure of lung epithelia to H2S decreased net transepithelial electrolyte absorption. This was due to an impairment of amiloride-sensitive sodium transport. H2S inhibited the activity of the Na+/K+-ATPase as well as lidocaine-sensitive potassium channels located in the basolateral membrane of the epithelium. Inhibition of these transport molecules diminishes the electrochemical gradient which is necessary for transepithelial sodium absorption. Since sodium absorption osmotically facilitates alveolar fluid clearance, interference of H2S with the epithelial transport machinery provides a mechanism which enhances edema formation in H2S-exposed lungs.
Conflict of interest statement
Figures


Similar articles
-
Hydrogen sulfide decreases β-adrenergic agonist-stimulated lung liquid clearance by inhibiting ENaC-mediated transepithelial sodium absorption.Am J Physiol Regul Integr Comp Physiol. 2015 Apr 1;308(7):R636-49. doi: 10.1152/ajpregu.00489.2014. Epub 2015 Jan 28. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25632025 Free PMC article.
-
The gasotransmitter hydrogen sulphide decreases Na⁺ transport across pulmonary epithelial cells.Br J Pharmacol. 2012 Jul;166(6):1946-63. doi: 10.1111/j.1476-5381.2012.01909.x. Br J Pharmacol. 2012. PMID: 22352810 Free PMC article.
-
Hydrogen sulfide contributes to hypoxic inhibition of airway transepithelial sodium absorption.Am J Physiol Regul Integr Comp Physiol. 2016 Sep 1;311(3):R607-17. doi: 10.1152/ajpregu.00177.2016. Epub 2016 Jul 20. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27440715
-
Transepithelial sodium and water transport in the lung. Major player and novel therapeutic target in pulmonary edema.Adv Exp Med Biol. 2001;502:315-38. doi: 10.1007/978-1-4757-3401-0_21. Adv Exp Med Biol. 2001. PMID: 11950147 Review.
-
Alveolar epithelial fluid transport in acute lung injury: new insights.Eur Respir J. 2002 Nov;20(5):1299-313. doi: 10.1183/09031936.02.00401602. Eur Respir J. 2002. PMID: 12449188 Review.
Cited by
-
Hydrogen sulfide as a regulator of respiratory epithelial sodium transport: the role of sodium-potassium ATPase. Focus on "Hydrogen sulfide contributes to hypoxic inhibition of airway transepithelial sodium absorption".Am J Physiol Regul Integr Comp Physiol. 2016 Sep 1;311(3):R564-5. doi: 10.1152/ajpregu.00327.2016. Epub 2016 Aug 3. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27488892 Free PMC article. No abstract available.
-
An emerging role for gasotransmitters in the control of breathing and ionic regulation in fish.J Comp Physiol B. 2016 Feb;186(2):145-59. doi: 10.1007/s00360-015-0949-x. Epub 2015 Dec 11. J Comp Physiol B. 2016. PMID: 26660653 Review.
-
Epithelial Electrolyte Transport Physiology and the Gasotransmitter Hydrogen Sulfide.Oxid Med Cell Longev. 2016;2016:4723416. doi: 10.1155/2016/4723416. Epub 2016 Jan 20. Oxid Med Cell Longev. 2016. PMID: 26904165 Free PMC article. Review.
-
Hydrogen sulfide stimulates CFTR in Xenopus oocytes by activation of the cAMP/PKA signalling axis.Sci Rep. 2017 Jun 14;7(1):3517. doi: 10.1038/s41598-017-03742-5. Sci Rep. 2017. PMID: 28615646 Free PMC article.
-
Hydrogen sulfide decreases β-adrenergic agonist-stimulated lung liquid clearance by inhibiting ENaC-mediated transepithelial sodium absorption.Am J Physiol Regul Integr Comp Physiol. 2015 Apr 1;308(7):R636-49. doi: 10.1152/ajpregu.00489.2014. Epub 2015 Jan 28. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25632025 Free PMC article.
References
-
- Guidotti TL (1996) Hydrogen sulphide. Occup Med (Lond) 46 5: 367–371. - PubMed
-
- Mimoun S, Andriamihaja M, Chaumontet C, Atanasiu C, Benamouzig R, et al. (2012) Detoxification of H(2)S by differentiated colonic epithelial cells: implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid Redox Signal 17 1: 1–10. - PubMed
-
- Guidotti TL (2010) Hydrogen sulfide: advances in understanding human toxicity. Int J Toxicol 29 6: 569–581. - PubMed
-
- Fronius M (2013) Treatment of pulmonary edema by ENaC activators/stimulators. Curr Mol Pharmacol 6 1: 13–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

