tLivin displays flexibility by promoting alternative cell death mechanisms
- PMID: 24960127
- PMCID: PMC4069184
- DOI: 10.1371/journal.pone.0101075
tLivin displays flexibility by promoting alternative cell death mechanisms
Abstract
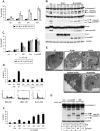
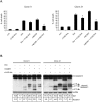
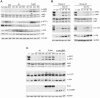
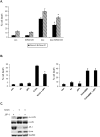
Livin is a member of the Inhibitor of Apoptosis (IAP) protein family that inhibits apoptosis triggered by a variety of stimuli. We previously demonstrated that while Livin inhibits caspase activity, caspases can cleave Livin to produce a truncated protein, tLivin and that this newly formed tLivin paradoxically induces cell death. However to date, the mechanism of tLivin-induced cell death is not fully understood. In this study, we set out to characterize the form of cell death mediated by tLivin. Here we demonstrate that, unlike most death-promoting proteins, tLivin is a flexible inducer of cell death capable of promoting necrosis or apoptosis in different cell lines. The unusual flexibility of tLivin is displayed by its ability to activate an alternative form of cell death when apoptosis is inhibited. Thus, tLivin can promote more than one form of cell death in the same cell type. Interestingly, in cells where tLivin induces necrosis, deletion of the caspase binding BIR domain results in tLivin-induced apoptosis, suggesting the BIR domain can potentially hamper the ability of tLivin to induce apoptosis. We further elucidate that tLivin activates the JNK pathway and both tLivin-induced apoptosis and necrosis are partially mediated by JNK activity. Acquired resistance to apoptosis, common in many tumors, impinges on the efficiency of conventional anti-cancer agents that function primarily by inducing apoptosis. The ability of tLivin to induce death of apoptosis-compromised cells makes it an attractive candidate for targeted cancer therapy.
Conflict of interest statement
Figures


References
-
- Assuncao Guimaraes C, Linden R (2004) Programmed cell deaths. Apoptosis and alternative deathstyles. Eur J Biochem 271: 1638–1650. - PubMed
-
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11: 700–714. - PubMed
-
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4: 387–396. - PubMed
-
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, et al. (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1: 489–495. - PubMed
-
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, et al. (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297: 259–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

