Chronic inflammation induces telomere dysfunction and accelerates ageing in mice
- PMID: 24960204
- PMCID: PMC4090717
- DOI: 10.1038/ncomms5172
Chronic inflammation induces telomere dysfunction and accelerates ageing in mice
Abstract
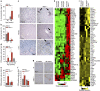
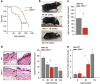
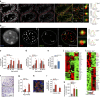
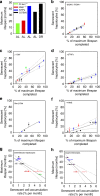
Chronic inflammation is associated with normal and pathological ageing. Here we show that chronic, progressive low-grade inflammation induced by knockout of the nfkb1 subunit of the transcription factor NF-κB induces premature ageing in mice. We also show that these mice have reduced regeneration in liver and gut. nfkb1(-/-) fibroblasts exhibit aggravated cell senescence because of an enhanced autocrine and paracrine feedback through NF-κB, COX-2 and ROS, which stabilizes DNA damage. Preferential accumulation of telomere-dysfunctional senescent cells in nfkb1(-/-) tissues is blocked by anti-inflammatory or antioxidant treatment of mice, and this rescues tissue regenerative potential. Frequencies of senescent cells in liver and intestinal crypts quantitatively predict mean and maximum lifespan in both short- and long-lived mice cohorts. These data indicate that systemic chronic inflammation can accelerate ageing via ROS-mediated exacerbation of telomere dysfunction and cell senescence in the absence of any other genetic or environmental factor.
Figures


References
-
- Franceschi C. et al.. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0700890/MRC_/Medical Research Council/United Kingdom
- BB/I020748/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H022384/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0900535/MRC_/Medical Research Council/United Kingdom
- MK/K001949/1/MRC_/Medical Research Council/United Kingdom
- G0700718/MRC_/Medical Research Council/United Kingdom
- WT084961MA/WT_/Wellcome Trust/United Kingdom
- MR/K001949/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/L016354/1/MRC_/Medical Research Council/United Kingdom
- NC/K000748/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- BB/C008200/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F010966/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

