A novel strain of porcine adenovirus detected in urinary bladder urothelial cell culture
- PMID: 24960273
- PMCID: PMC4074940
- DOI: 10.3390/v6062505
A novel strain of porcine adenovirus detected in urinary bladder urothelial cell culture
Abstract
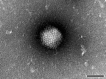
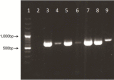
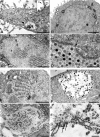
Contamination of cell cultures is the most common problem encountered in cell culture laboratories. Besides the secondary cell contaminations often occurring in the cell laboratories, the contaminations originating from donor animal or human tissue are equally as common, but usually harder to recognize and as such require special attention. The present study describes the detection of porcine adenovirus (PAdV), strain PAdV-SVN1 in cultures of normal porcine urothelial (NPU) cells isolated from urinary bladders of domestic pigs. NPU cell cultures were evaluated by light microscopy (LM), polymerase chain reaction (PCR), and additionally assessed by transmission electron microscopy (TEM). Characteristic ultrastructure of virions revealed the infection with adenovirus. The adenoviral contamination was further identified by the sequence analysis, which showed the highest similarity to recently described PAdV strain PAdV-WI. Additionally, the cell ultrastructural analysis confirmed the life-cycle characteristic for adenoviruses. To closely mimic the in vivo situation, the majority of research on in vitro models uses cell cultures isolated from human or animal tissue and their subsequent passages. Since the donor tissue could be a potential source of contamination, the microbiological screening of the excised tissue and harvested cell cultures is highly recommended.
Figures


Similar articles
-
Molecular epidemiology and characterization of porcine adenoviruses in pigs with diarrhea in Thailand.Infect Genet Evol. 2019 Jan;67:73-77. doi: 10.1016/j.meegid.2018.10.026. Epub 2018 Nov 2. Infect Genet Evol. 2019. PMID: 30391718
-
Detection of bovine and porcine adenoviruses for tracing the source of fecal contamination.Appl Environ Microbiol. 2004 Mar;70(3):1448-54. doi: 10.1128/AEM.70.3.1448-1454.2004. Appl Environ Microbiol. 2004. PMID: 15006765 Free PMC article.
-
F-coliphages, porcine adenovirus and porcine teschovirus as potential indicator viruses of fecal contamination for pork carcass processing.Int J Food Microbiol. 2017 Jan 16;241:237-243. doi: 10.1016/j.ijfoodmicro.2016.10.032. Epub 2016 Oct 28. Int J Food Microbiol. 2017. PMID: 27810445
-
Porcine adenovirus as a delivery system for swine vaccines and immunotherapeutics.Vet J. 2005 Jan;169(1):17-27. doi: 10.1016/j.tvjl.2003.09.007. Vet J. 2005. PMID: 15683761 Free PMC article. Review.
-
The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--chapter 5: Strategies to prevent transmission of porcine endogenous retroviruses.Xenotransplantation. 2009 Jul-Aug;16(4):239-48. doi: 10.1111/j.1399-3089.2009.00544.x. Xenotransplantation. 2009. PMID: 19799764
Cited by
-
High Rates of Detection and Molecular Characterization of Porcine Adenovirus Serotype 5 (Porcine mastadenovirus C) from Diarrheic Pigs.Pathogens. 2022 Oct 20;11(10):1210. doi: 10.3390/pathogens11101210. Pathogens. 2022. PMID: 36297267 Free PMC article.
-
A novel adenovirus isolated from the Egyptian fruit bat in South Africa is closely related to recent isolates from China.Sci Rep. 2018 Jun 25;8(1):9584. doi: 10.1038/s41598-018-27836-w. Sci Rep. 2018. PMID: 29942032 Free PMC article.
-
Attachment of Cancer Urothelial Cells to the Bladder Epithelium Occurs on Uroplakin-Negative Cells and Is Mediated by Desmosomal and Not by Classical Cadherins.Int J Mol Sci. 2021 May 25;22(11):5565. doi: 10.3390/ijms22115565. Int J Mol Sci. 2021. PMID: 34070317 Free PMC article.
-
Characterization of the First Genome of Porcine mastadenovirus B (HNU1 Strain) and Implications on Its Lymphoid and Special Origin.Virol Sin. 2020 Oct;35(5):528-537. doi: 10.1007/s12250-020-00210-9. Epub 2020 Mar 31. Virol Sin. 2020. PMID: 32236817 Free PMC article.
-
Human amniotic membrane inhibits migration and invasion of muscle-invasive bladder cancer urothelial cells by downregulating the FAK/PI3K/Akt/mTOR signalling pathway.Sci Rep. 2023 Nov 6;13(1):19227. doi: 10.1038/s41598-023-46091-2. Sci Rep. 2023. PMID: 37932474 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

