Eosinophil-associated lung diseases. A cry for surfactant proteins A and D help?
- PMID: 24960334
- PMCID: PMC4224083
- DOI: 10.1165/rcmb.2014-0095TR
Eosinophil-associated lung diseases. A cry for surfactant proteins A and D help?
Abstract
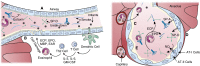
Surfactant proteins (SP)-A and SP-D (SP-A/-D) play important roles in numerous eosinophil-dominated diseases, including asthma, allergic bronchopulmonary aspergillosis, and allergic rhinitis. In these settings, SP-A/-D have been shown to modulate eosinophil chemotaxis, inhibit eosinophil mediator release, and mediate macrophage clearance of apoptotic eosinophils. Dysregulation of SP-A/-D function in eosinophil-dominated diseases is also not uncommon. Alterations in serum SP-A/-D levels are associated with disease severity in allergic rhinitis and chronic obstructive pulmonary disease. Furthermore, oligimerization of SP-A/-D, necessary for their proper function, can be perturbed by reactive nitrogen species, which are increased in eosinophilic disease. In this review, we highlight the associations of eosinophilic lung diseases with SP-A and SP-D levels and functions.
Keywords: collectin; eosinophil; surfactant; surfactant protein-A; surfactant protein-D.
Figures
References
-
- Hakansson K, Lim NK, Hoppe HJ, Reid KB. Crystal structure of the trimeric alpha-helical coiled-coil and the three lectin domains of human lung surfactant protein D. Structure. 1999;7:255–264. - PubMed
-
- Crouch E, Persson A, Chang D, Heuser J. Molecular structure of pulmonary surfactant protein D (SP-D) J Biol Chem. 1994;269:17311–17319. - PubMed
-
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. - PubMed
-
- Winkler C, Hohlfeld JM. Surfactant and allergic airway inflammation. Swiss Med Wkly. 2013;143:w13818. - PubMed
-
- LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, Korfhagen T. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31:193–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

