α-Synuclein-induced membrane remodeling is driven by binding affinity, partition depth, and interleaflet order asymmetry
- PMID: 24960410
- PMCID: PMC4105054
- DOI: 10.1021/ja5016958
α-Synuclein-induced membrane remodeling is driven by binding affinity, partition depth, and interleaflet order asymmetry
Abstract
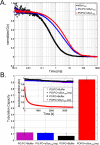
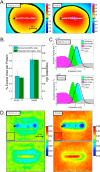
We have investigated the membrane remodeling capacity of the N-terminal membrane-binding domain of α-synuclein (α-Syn100). Using fluorescence correlation spectroscopy and vesicle clearance assays, we show that α-Syn100 fully tubulates POPG vesicles, the first demonstration that the amphipathic helix on its own is capable of this effect. We also show that at equal density of membrane-bound protein, α-Syn has dramatically reduced affinity for, and does not tubulate, vesicles composed of a 1:1 POPG:POPC mixture. Coarse-grained molecular dynamics simulations suggested that the difference between the pure POPG and mixture results may be attributed to differences in the protein's partition depth, the membrane's hydrophobic thickness, and disruption of acyl chain order. To explore the importance of these attributes compared with the role of the reduced binding energy, we created an α-Syn100 variant in which we removed the hydrophobic core of the non-amyloid component (NAC) domain and tested its impact on pure POPG vesicles. We observed a substantial reduction in binding affinity and tubulation, and simulations of the NAC-null protein suggested that the reduced binding energy increases the protein mobility on the bilayer surface, likely impacting the protein's ability to assemble into organized pretubule structures. We also used simulations to explore a potential role for interleaflet coupling as an additional driving force for tubulation. We conclude that symmetry across the leaflets in the tubulated state maximizes the interaction energy of the two leaflets and relieves the strain induced by the hydrophobic void beneath the amphipathic helix.
Figures





References
-
- Robotta M.; Braun P.; van Rooijen B.; Subramaniam V.; Huber M.; Drescher M. ChemPhysChem 2011, 12, 267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

