Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy
- PMID: 24960415
- PMCID: PMC4128341
- DOI: 10.1667/RR13374.1
Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy
Abstract
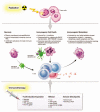
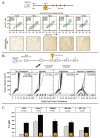
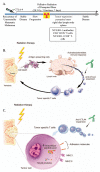
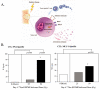
There is increasing evidence of the potential for radiation therapy to generate antitumor immune responses. The mechanisms of this immune-activating potential include actions on tumor cells such as immunogenic cell death and phenotypic change. Radiation modulates tumor cell surface expression of cell death receptors, tumor-associated antigens and adhesion molecules. This process of immunomodulation sensitizes tumor cells to immune-mediated killing. Radiation also affects immune compartments, including antigen-presenting cells, cytotoxic T lymphocytes and humoral immunity, leading to specific antitumor immune responses. Recognizing the importance of immunity as a potentiator of response to radiation leads to rational augmentation of antitumor immunity by combining radiation and immunotherapy. Targeted immunotherapy manipulates the immune system in a way that best synergizes with radiation. This article discusses the ability of radiation monotherapy to induce antitumor immunity, with a focus on the effect of radiation on antigen-presenting cells and cytotoxic T lymphocytes. We define two important responses generated by tumor cells, immunogenic cell death and immunomodulation, both of which are radiation dose-dependent. In conclusion, we describe the translation of several combination therapies from the preclinical to the clinical setting and identify opportunities for further exploration.
Figures

References
-
- Smith BD, Haffty BG, Wilson LD, Smith GL, Patel AN, Buchholz TA. The future of radiation oncology in the United States from 2010 to 2020: will supply keep pace with demand? J Clin Oncol. 2010;28:5160–5. - PubMed
-
- Peters ME, Shareef MM, Gupta S, Zagurovskaya-Sultanov M, Kadhim M, Mohiuddin M, et al. Potential utilization of bystander/abscopal-mediated signal transduction events in the treatment of solid tumors. Curr Signal TransductTherapy. 2007;2:129–43.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

