NADPH oxidase and angiogenesis following endothelin-1 induced stroke in rats: role for nox2 in brain repair
- PMID: 24961316
- PMCID: PMC4061826
- DOI: 10.3390/brainsci3010294
NADPH oxidase and angiogenesis following endothelin-1 induced stroke in rats: role for nox2 in brain repair
Abstract
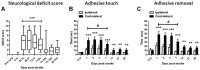
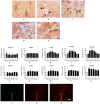
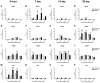
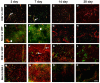
NADPH oxidases contribute to brain injury, yet they may also have a role in brain repair, particularly in vascular signaling and angiogenesis. This study determined the temporal and spatial profile of NADPH oxidase subunit expression/activity concurrently with angiogenesis in the brain following transient ischemic stroke induced by prolonged constriction of the middle cerebral artery by perivascular injection of endothelin-1 in conscious Hooded Wistar rats (n = 47). VEGF mRNA expression was increased in the ipsilateral cortex and striatum between 6 h and 28 days post-stroke concurrently with a marked increase in Nox2 mRNA expression up to 7 days, and increased Nox4 mRNA expression detected between 7 and 28 days. Point counting of blood vessels using Metamorph imaging software showed increased vascular sprouting between 3 and 7 days after stroke with new vascular networks detected in the core infarct region by 14 days. Angiogenic blood vessels 3 and 7 days post-stroke were observed to co-localise with both Nox2 antibody and dihydroethidium fluorescence suggesting a role for Nox2 generated superoxide during the phase of vascular remodeling, whilst Nox4 expression was detected once new cerebral vessels had formed. These results indicate for the first time that ROS signaling through a cerebrovascular Nox2 NADPH oxidase may be important in initiating brain angiogenesis.
Figures




References
-
- Zhang R., Zhang Z., Wang L., Wang Y., Gousev A., Zhang L., Ho K.L., Morshead C., Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J. Cereb. Blood Flow Metab. 2004;2:441–448. - PubMed
-
- Wang L., Zhang Z., Wang Y., Zhang R., Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2007;35:1732–1737. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

