Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells
- PMID: 24961503
- PMCID: PMC4276551
- DOI: 10.1177/1470320313515039
Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells
Abstract
Introduction: Isolated nuclei of sheep proximal tubules express angiotensin (Ang) receptors as well as angiotensinogen (AGT) and renin. The present study characterized the NRK-52E tubular epithelial cell line for the intracellular expression of renin-angiotensin system (RAS) components.
Methods: RAS components were visualized by immunofluorescent staining in intact cells and protein expression in isolated nuclei.
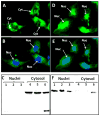
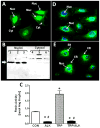
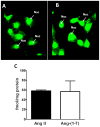
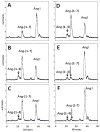
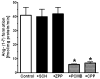
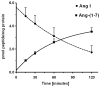
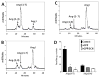
Results: An antibody to the angiotensin I (Ang I) sequence of AGT (AI-AGT) revealed only cytosolic staining, while an antibody to an internal sequence of AGT (Int-AGT) revealed primarily nuclear staining. Immunoblots of nuclear and cytosolic fractions confirmed the differential cell staining of AGT. Immunostaining for renin was present on nuclei of intact cells. Nuclear renin activity averaged 0.77±0.05 nmol/mg protein/h that was reduced by aliskiren (0.13±0.01 nmol/mg/h, n=3, p<0.01); trypsin activation increased activity three-fold. Peptide staining localized angiotensin II (Ang II) and Ang-(1-7) to the nucleus and peptide content averaged 59±2 and 57±22 fmol/mg (n=4), respectively. Peptide metabolism in isolated nuclei revealed the processing of Ang I to Ang-(1-7) by thimet oligopeptidase.
Conclusion: We conclude that the NRK-52E cells express an intracellular RAS localized to the nucleus and may be an appropriate cell model to elucidate the functional relevance of this system.
Keywords: NRK-52E; angiotensin; angiotensinogen; renin; thimet oligopeptidase.
© The Author(s) 2014.
Conflict of interest statement
None declared
Figures

References
-
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–87. - PubMed
-
- Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. - PubMed
-
- Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol. 2009;5(2):89–100. - PubMed
-
- Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294(4):H1675–1684. - PubMed
-
- Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293(2):H939–H948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

