White matter loss in a mouse model of periventricular leukomalacia is rescued by trophic factors
- PMID: 24961618
- PMCID: PMC4061895
- DOI: 10.3390/brainsci3041461
White matter loss in a mouse model of periventricular leukomalacia is rescued by trophic factors
Abstract
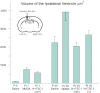
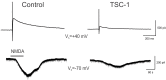
Periventricular leukomalacia (PVL) is the most frequent cause of cerebral palsy and other intellectual disabilities, and currently there is no treatment. In PVL, glutamate excitotoxicity (GME) leads to abnormal oligodendrocytes (OLs), myelin deficiency, and ventriculomegaly. We have previously identified that the combination of transferrin and insulin growth factors (TSC1) promotes endogenous OL regeneration and remyelination in the postnatal and adult rodent brain. Here, we produced a periventricular white matter lesion with a single intracerebral injection of N-methyl-d-aspartate (NMDA). Comparing lesions produced by NMDA alone and those produced by NMDA + TSC1 we found that: NMDA affected survival and reduced migration of OL progenitors (OLPs). In contrast, mice injected with NMDA + TSC1 proliferated twice as much indicating that TSC1 supported regeneration of the OLP population after the insult. Olig2-mRNA expression showed 52% OLP survival in mice receiving a NMDA injection and increased to 78% when TSC1 + NMDA were injected simultaneously and ventricular size was reduced by TSC1. Furthermore, in striatal slices TSC1 reduced the inward currents induced by NMDA in medium-sized spiny neurons, demonstrating neuroprotection. Thus, white matter loss after excitotoxicity can be partially rescued as TSC1 conferred neuroprotection to preexisting OLP and regeneration via OLP proliferation. Furthermore, we showed that early TSC1 administration maximizes neuroprotection.
Figures





Similar articles
-
Trophic factors are essential for the survival of grafted oligodendrocyte progenitors and for neuroprotection after perinatal excitotoxicity.Neural Regen Res. 2020 Mar;15(3):557-568. doi: 10.4103/1673-5374.266066. Neural Regen Res. 2020. PMID: 31571668 Free PMC article.
-
Trophic factors intervention regenerates the nestin-expressing cell population in a model of perinatal excitotoxicity: Implications for perinatal brain injury and prematurity.Integr Mol Med. 2016 Jun;3(3):703-715. doi: 10.15761/imm.1000228. Epub 2016 Jun 25. Integr Mol Med. 2016. PMID: 35558521 Free PMC article.
-
Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury.J Neurosci. 2001 Feb 15;21(4):1302-12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. J Neurosci. 2001. PMID: 11160401 Free PMC article.
-
Neuropathologic substrate of cerebral palsy.J Child Neurol. 2005 Dec;20(12):940-9. doi: 10.1177/08830738050200120301. J Child Neurol. 2005. PMID: 16417840 Review.
-
Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system.Neuropathology. 2002 Sep;22(3):106-32. doi: 10.1046/j.1440-1789.2002.00438.x. Neuropathology. 2002. PMID: 12416551 Review.
Cited by
-
Systems approach to the study of brain damage in the very preterm newborn.Front Syst Neurosci. 2015 Apr 14;9:58. doi: 10.3389/fnsys.2015.00058. eCollection 2015. Front Syst Neurosci. 2015. PMID: 25926780 Free PMC article.
-
Delayed Maturation of Oligodendrocyte Progenitors by Microgravity: Implications for Multiple Sclerosis and Space Flight.Life (Basel). 2022 May 27;12(6):797. doi: 10.3390/life12060797. Life (Basel). 2022. PMID: 35743828 Free PMC article.
-
Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration.Neurochem Res. 2016 Feb;41(1-2):431-49. doi: 10.1007/s11064-016-1850-z. Epub 2016 Feb 17. Neurochem Res. 2016. PMID: 26883642 Free PMC article.
-
Pharmacological approaches to intervention in hypomyelinating and demyelinating white matter pathology.Neuropharmacology. 2016 Nov;110(Pt B):605-625. doi: 10.1016/j.neuropharm.2015.06.008. Epub 2015 Jun 24. Neuropharmacology. 2016. PMID: 26116759 Free PMC article. Review.
-
Trophic factors are essential for the survival of grafted oligodendrocyte progenitors and for neuroprotection after perinatal excitotoxicity.Neural Regen Res. 2020 Mar;15(3):557-568. doi: 10.4103/1673-5374.266066. Neural Regen Res. 2020. PMID: 31571668 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

