Towards effective non-invasive brain-computer interfaces dedicated to gait rehabilitation systems
- PMID: 24961699
- PMCID: PMC4066236
- DOI: 10.3390/brainsci4010001
Towards effective non-invasive brain-computer interfaces dedicated to gait rehabilitation systems
Abstract
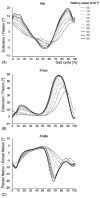
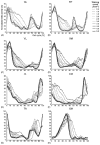
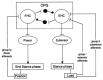
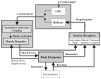
In the last few years, significant progress has been made in the field of walk rehabilitation. Motor cortex signals in bipedal monkeys have been interpreted to predict walk kinematics. Epidural electrical stimulation in rats and in one young paraplegic has been realized to partially restore motor control after spinal cord injury. However, these experimental trials are far from being applicable to all patients suffering from motor impairments. Therefore, it is thought that more simple rehabilitation systems are desirable in the meanwhile. The goal of this review is to describe and summarize the progress made in the development of non-invasive brain-computer interfaces dedicated to motor rehabilitation systems. In the first part, the main principles of human locomotion control are presented. The paper then focuses on the mechanisms of supra-spinal centers active during gait, including results from electroencephalography, functional brain imaging technologies [near-infrared spectroscopy (NIRS), functional magnetic resonance imaging (fMRI), positron-emission tomography (PET), single-photon emission-computed tomography (SPECT)] and invasive studies. The first brain-computer interface (BCI) applications to gait rehabilitation are then presented, with a discussion about the different strategies developed in the field. The challenges to raise for future systems are identified and discussed. Finally, we present some proposals to address these challenges, in order to contribute to the improvement of BCI for gait rehabilitation.
Figures




Similar articles
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
-
Non-Invasive Systems Application in Traumatic Brain Injury Rehabilitation.Brain Sci. 2023 Nov 15;13(11):1594. doi: 10.3390/brainsci13111594. Brain Sci. 2023. PMID: 38002552 Free PMC article. Review.
-
Rehabilitation of gait after stroke: a review towards a top-down approach.J Neuroeng Rehabil. 2011 Dec 13;8:66. doi: 10.1186/1743-0003-8-66. J Neuroeng Rehabil. 2011. PMID: 22165907 Free PMC article. Review.
-
Gait adaptation to visual kinematic perturbations using a real-time closed-loop brain-computer interface to a virtual reality avatar.J Neural Eng. 2016 Jun;13(3):036006. doi: 10.1088/1741-2560/13/3/036006. Epub 2016 Apr 11. J Neural Eng. 2016. PMID: 27064824 Free PMC article.
-
The clinical effects of brain-computer interface with robot on upper-limb function for post-stroke rehabilitation: a meta-analysis and systematic review.Disabil Rehabil Assist Technol. 2024 Jan;19(1):30-41. doi: 10.1080/17483107.2022.2060354. Epub 2022 Apr 21. Disabil Rehabil Assist Technol. 2024. PMID: 35450498
Cited by
-
Subthalamic oscillatory activity and connectivity during gait in Parkinson's disease.Neuroimage Clin. 2018 May 3;19:396-405. doi: 10.1016/j.nicl.2018.05.001. eCollection 2018. Neuroimage Clin. 2018. PMID: 30035024 Free PMC article.
-
Human-Robot Interaction: Does Robotic Guidance Force Affect Gait-Related Brain Dynamics during Robot-Assisted Treadmill Walking?PLoS One. 2015 Oct 20;10(10):e0140626. doi: 10.1371/journal.pone.0140626. eCollection 2015. PLoS One. 2015. PMID: 26485148 Free PMC article.
-
The role of virtual reality in improving motor performance as revealed by EEG: a randomized clinical trial.J Neuroeng Rehabil. 2017 Jun 7;14(1):53. doi: 10.1186/s12984-017-0268-4. J Neuroeng Rehabil. 2017. PMID: 28592282 Free PMC article. Clinical Trial.
-
TechnoBrainBodies-in-Cultures: An Intersectional Case.Front Sociol. 2021 Apr 27;6:651486. doi: 10.3389/fsoc.2021.651486. eCollection 2021. Front Sociol. 2021. PMID: 33987221 Free PMC article.
-
Analysis of the Relationship Between Motor Imagery and Age-Related Fatigue for CNN Classification of the EEG Data.Front Aging Neurosci. 2022 Jul 14;14:909571. doi: 10.3389/fnagi.2022.909571. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35912081 Free PMC article.
References
-
- Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.V.E. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet. 2011;377:1938–1947. - PMC - PubMed
-
- Cheron G., Bengoetxea A., Bouillot E., Lacquaniti F., Dan B. Early emergence of temporal co-ordination of lower limb segments elevation angles in human locomotion. Neurosci. Lett. 2001;308:123–127. - PubMed
-
- Cheron G., Bouillot E., Dan B., Bengoetxea A., Draye J., Lacquaniti F. Development of a kinematic coordination pattern in toddler locomotion: Planar covariation. Exp. Brain Res. 2001;137:455–466. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

