Heterogeneity and plasticity of epidermal stem cells
- PMID: 24961797
- PMCID: PMC4067958
- DOI: 10.1242/dev.104588
Heterogeneity and plasticity of epidermal stem cells
Abstract
The epidermis is an integral part of our largest organ, the skin, and protects us against the hostile environment. It is a highly dynamic tissue that, during normal steady-state conditions, undergoes constant turnover. Multiple stem cell populations residing in autonomously maintained compartments facilitate this task. In this Review, we discuss stem cell behaviour during normal tissue homeostasis, regeneration and disease within the pilosebaceous unit, an integral structure of the epidermis that is responsible for hair growth and lubrication of the epithelium. We provide an up-to-date view of the pilosebaceous unit, encompassing the heterogeneity and plasticity of multiple discrete stem cell populations that are strongly influenced by external cues to maintain their identity and function.
Keywords: Epidermis; Regeneration; Stem cells; Tissue homeostasis.
© 2014. Published by The Company of Biologists Ltd.
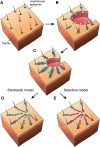
Figures


References
-
- Agrawal N., Frederick M. J., Pickering C. R., Bettegowda C., Chang K., Li R. J., Fakhry C., Xie T.-X., Zhang J., Wang J., et al. (2011). Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154-1157 10.1126/science.1206923 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

