Macrophages modulate adult zebrafish tail fin regeneration
- PMID: 24961798
- PMCID: PMC4067955
- DOI: 10.1242/dev.098459
Macrophages modulate adult zebrafish tail fin regeneration
Erratum in
- Development. 2015 Jan 15;142(2):406. Tsung-Yang, Chao [corrected to Yang, Chao-Tsung]
Abstract
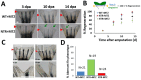
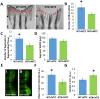
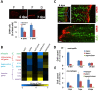
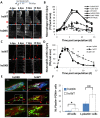
Neutrophils and macrophages, as key mediators of inflammation, have defined functionally important roles in mammalian tissue repair. Although recent evidence suggests that similar cells exist in zebrafish and also migrate to sites of injury in larvae, whether these cells are functionally important for wound healing or regeneration in adult zebrafish is unknown. To begin to address these questions, we first tracked neutrophils (lyzC(+), mpo(+)) and macrophages (mpeg1(+)) in adult zebrafish following amputation of the tail fin, and detailed a migratory timecourse that revealed conserved elements of the inflammatory cell response with mammals. Next, we used transgenic zebrafish in which we could selectively ablate macrophages, which allowed us to investigate whether macrophages were required for tail fin regeneration. We identified stage-dependent functional roles of macrophages in mediating fin tissue outgrowth and bony ray patterning, in part through modulating levels of blastema proliferation. Moreover, we also sought to detail molecular regulators of inflammation in adult zebrafish and identified Wnt/β-catenin as a signaling pathway that regulates the injury microenvironment, inflammatory cell migration and macrophage phenotype. These results provide a cellular and molecular link between components of the inflammation response and regeneration in adult zebrafish.
Keywords: Fin; Inflammation; Macrophages; Neutrophils; Regeneration; Wnt; Zebrafish.
© 2014. Published by The Company of Biologists Ltd.
Figures



References
-
- Cailhier J. F., Sawatzky D. A., Kipari T., Houlberg K., Walbaum D., Watson S., Lang R. A., Clay S., Kluth D., Savill J., et al. (2006). Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation. Am. J. Respir. Crit. Care Med. 173, 540-547 10.1164/rccm.200504-538OC - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

