Gene expression profiling identifies the zinc-finger protein Charlatan as a regulator of intestinal stem cells in Drosophila
- PMID: 24961799
- PMCID: PMC4067959
- DOI: 10.1242/dev.106237
Gene expression profiling identifies the zinc-finger protein Charlatan as a regulator of intestinal stem cells in Drosophila
Abstract
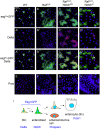
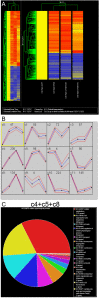
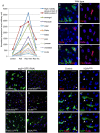
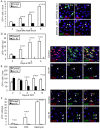
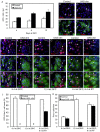
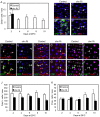
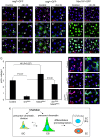
Intestinal stem cells (ISCs) in the adult Drosophila midgut can respond to tissue damage and support repair. We used genetic manipulation to increase the number of ISC-like cells in the adult midgut and performed gene expression profiling to identify potential ISC regulators. A detailed analysis of one of these potential regulators, the zinc-finger protein Charlatan, was carried out. MARCM clonal analysis and RNAi in precursor cells showed that loss of Chn function caused severe ISC division defects, including loss of EdU incorporation, phosphorylated histone 3 staining and expression of the mitotic protein Cdc2. Loss of Charlatan also led to a much reduced histone acetylation staining in precursor cells. Both the histone acetylation and ISC division defects could be rescued by the simultaneous decrease of the Histone Deacetylase 2. The overexpression of Charlatan blocked differentiation reversibly, but loss of Charlatan did not lead to automatic differentiation. The results together suggest that Charlatan does not simply act as an anti-differentiation factor but instead functions to maintain a chromatin structure that is compatible with stem cell properties, including proliferation.
Keywords: Charlatan; Chromatin; Drosophila; Intestine; Stem cells; Zinc-finger protein.
© 2014. Published by The Company of Biologists Ltd.
Figures
Similar articles
-
Nipped-A regulates intestinal stem cell proliferation in Drosophila.Development. 2017 Feb 15;144(4):612-623. doi: 10.1242/dev.142703. Development. 2017. PMID: 28196804
-
The Osa-containing SWI/SNF chromatin-remodeling complex regulates stem cell commitment in the adult Drosophila intestine.Development. 2013 Sep;140(17):3532-40. doi: 10.1242/dev.096891. Development. 2013. PMID: 23942514 Free PMC article.
-
Transcriptional control of stem cell maintenance in the Drosophila intestine.Development. 2010 Mar;137(5):705-14. doi: 10.1242/dev.039404. Development. 2010. PMID: 20147375 Free PMC article.
-
Intestinal stem cell asymmetric division in the Drosophila posterior midgut.J Cell Physiol. 2010 Sep;224(3):581-4. doi: 10.1002/jcp.22194. J Cell Physiol. 2010. PMID: 20578235 Review.
-
Genetic mechanisms regulating stem cell self-renewal and differentiation in the central nervous system of Drosophila.Cell Adh Migr. 2009 Oct-Dec;3(4):402-11. doi: 10.4161/cam.3.4.8690. Epub 2009 Oct 7. Cell Adh Migr. 2009. PMID: 19421003 Free PMC article. Review.
Cited by
-
Tissue-resident stem cell activity: a view from the adult Drosophila gastrointestinal tract.Cell Commun Signal. 2017 Sep 18;15(1):33. doi: 10.1186/s12964-017-0184-z. Cell Commun Signal. 2017. PMID: 28923062 Free PMC article. Review.
-
Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals.Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12218-12223. doi: 10.1073/pnas.1719169115. Epub 2018 Nov 7. Proc Natl Acad Sci U S A. 2018. PMID: 30404917 Free PMC article.
-
The WT1-like transcription factor Klumpfuss maintains lineage commitment of enterocyte progenitors in the Drosophila intestine.Nat Commun. 2019 Sep 11;10(1):4123. doi: 10.1038/s41467-019-12003-0. Nat Commun. 2019. PMID: 31511511 Free PMC article.
-
Asymmetric Centromeres Differentially Coordinate with Mitotic Machinery to Ensure Biased Sister Chromatid Segregation in Germline Stem Cells.Cell Stem Cell. 2019 Nov 7;25(5):666-681.e5. doi: 10.1016/j.stem.2019.08.014. Epub 2019 Sep 26. Cell Stem Cell. 2019. PMID: 31564548 Free PMC article.
-
Spen limits intestinal stem cell self-renewal.PLoS Genet. 2018 Nov 19;14(11):e1007773. doi: 10.1371/journal.pgen.1007773. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30452449 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

