Modulation of amyloid-β aggregation by histidine-coordinating Cobalt(III) Schiff base complexes
- PMID: 24961930
- PMCID: PMC4166533
- DOI: 10.1002/cbic.201402201
Modulation of amyloid-β aggregation by histidine-coordinating Cobalt(III) Schiff base complexes
Abstract
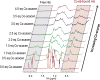
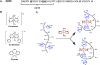
Oligomers of the Aβ42 peptide are significant neurotoxins linked to Alzheimer's disease (AD). Histidine (His) residues present at the N terminus of Aβ42 are believed to influence toxicity by either serving as metal-ion binding sites (which promote oligomerization and oxidative damage) or facilitating synaptic binding. Transition metal complexes that bind to these residues and modulate Aβ toxicity have emerged as therapeutic candidates. Cobalt(III) Schiff base complexes (Co-sb) were evaluated for their ability to interact with Aβ peptides. HPLC-MS, NMR, fluorescence, and DFT studies demonstrated that Co-sb complexes could interact with the His residues in a truncated Aβ16 peptide representing the Aβ42 N terminus. Coordination of Co-sb complexes altered the structure of Aβ42 peptides and promoted the formation of large soluble oligomers. Interestingly, this structural perturbation of Aβ correlated to reduced synaptic binding to hippocampal neurons. These results demonstrate the promise of Co-sb complexes in anti-AD therapeutic approaches.
Keywords: Alzheimer's disease; amyloid beta; cobalt; histidine; oligomers.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
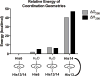


References
-
- Citron M. Nat Rev Drug Discovery. 2010;9:387–398. - PubMed
- Holtzman DM, John CM, Goate A. Sci Transl Med. 2011;3:77sr71. - PMC - PubMed
- Kenche VB, Barnham KJ. Br J Pharmacol. 2011;163:211–219. - PMC - PubMed
- Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde ALW, Jessen F, Hoessler YC, Sanhai WR, Zetterberg H, Woodcock J, Blennow K. Nat Rev Drug Discovery. 2010;9:560–574. - PubMed
-
- Ferreira ST, Klein WL. Neurobiol Learn Mem. 2011;96:529–543. - PMC - PubMed
- Hamley IW. Chem Rev. 2012;112:5147–5192. - PubMed
- Hung LW, Ciccotosto GD, Giannakis E, Tew DJ, Perez K, Masters CL, Cappai R, Wade JD, Barnham KJ. J Neurosci. 2008;28:11950–11958. - PMC - PubMed
- Klein WL. Alzheimer’s Dement. 2006;2:43–55. - PubMed
- Klein W, De Felice F, Lacor PN, Lambert MP, Zhao WQ. 2008:103–132.
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Proc Natl Acad Sci U S A. 1998;95:6448–6453. - PMC - PubMed
- Parodi J, Sepulveda FJ, Roa J, Opazo C, Inestrosa NC, Aguayo LG. J Biol Chem. 2010;285:2506–2514. - PMC - PubMed
- Rauk A. Chem Soc Rev. 2009;38:2698. - PubMed
- Shankar GM, Walsh DM. Molecular Neurodegeneration. 2009;4:48. - PMC - PubMed
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Biochem Soc Trans. 2002;30:552–557. - PubMed
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA. Brain Res. 2002;924:133–140. - PubMed
-
- Kim HJ, Chae SC, Lee DK, Chromy B, Lee SC. The FASEB Journal. 2003 - PubMed
- Rembach A, Ryan TM, Roberts BR, Doecke JD, Wilson WJ, Watt AD, Barnham KJ, Masters CL. Biomarkers in medicine. 2013;7:641–662. - PubMed
- Krafft GA, Klein WL. Neuropharmacology. 2010;59:230–242. - PubMed
- Lacor PN, Buniel MC, Furlow PW, Sanz Clemente A, Velasco PT, Wood M, Viola KL, Klein WL. J Neurosci. 2007;27:796–807. - PMC - PubMed
-
- Amijee H, Scopes D. J Alzheimer’s Dis. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

