Structural biology of TRP channels
- PMID: 24961976
- PMCID: PMC5075240
- DOI: 10.1007/978-3-319-05161-1_10
Structural biology of TRP channels
Abstract
Membrane proteins remain challenging targets for structural biologists, despite recent technical developments regarding sample preparation and structure determination. We review recent progress towards a structural understanding of TRP channels and the techniques used to that end. We discuss available low-resolution structures from electron microscopy (EM), X-ray crystallography, and nuclear magnetic resonance (NMR) and review the resulting insights into TRP channel function for various subfamily members. The recent high-resolution structure of TRPV1 is discussed in more detail in Chapter 11. We also consider the opportunities and challenges of using the accumulating structural information on TRPs and homologous proteins for deducing full-length structures of different TRP channel subfamilies, such as building homology models. Finally, we close by summarizing the outlook of the "holy grail" of understanding in atomic detail the diverse functions of TRP channels.
Figures
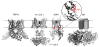

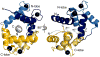

Similar articles
-
Structural biology of TRP channels.Adv Exp Med Biol. 2011;704:1-23. doi: 10.1007/978-94-007-0265-3_1. Adv Exp Med Biol. 2011. PMID: 21290287 Free PMC article.
-
Structural biology of thermoTRPV channels.Cell Calcium. 2019 Dec;84:102106. doi: 10.1016/j.ceca.2019.102106. Epub 2019 Nov 1. Cell Calcium. 2019. PMID: 31726322 Free PMC article. Review.
-
Dawning of a new era in TRP channel structural biology by cryo-electron microscopy.Pflugers Arch. 2018 Feb;470(2):213-225. doi: 10.1007/s00424-018-2107-2. Epub 2018 Jan 17. Pflugers Arch. 2018. PMID: 29344776 Review.
-
X-ray crystallography of TRP channels.Channels (Austin). 2018 Jan 1;12(1):137-152. doi: 10.1080/19336950.2018.1457898. Channels (Austin). 2018. PMID: 29589513 Free PMC article. Review.
-
Functional and structural studies of TRP channels heterologously expressed in budding yeast.Adv Exp Med Biol. 2011;704:25-40. doi: 10.1007/978-94-007-0265-3_2. Adv Exp Med Biol. 2011. PMID: 21290288 Free PMC article. Review.
Cited by
-
Permeating disciplines: Overcoming barriers between molecular simulations and classical structure-function approaches in biological ion transport.Biochim Biophys Acta Biomembr. 2018 Apr;1860(4):927-942. doi: 10.1016/j.bbamem.2017.12.013. Epub 2017 Dec 16. Biochim Biophys Acta Biomembr. 2018. PMID: 29258839 Free PMC article. Review.
-
The Molecular Biology of Placental Transport of Calcium to the Human Foetus.Int J Mol Sci. 2025 Jan 4;26(1):383. doi: 10.3390/ijms26010383. Int J Mol Sci. 2025. PMID: 39796238 Free PMC article. Review.
-
Structural Basis of TRPV4 N Terminus Interaction with Syndapin/PACSIN1-3 and PIP2.Structure. 2018 Dec 4;26(12):1583-1593.e5. doi: 10.1016/j.str.2018.08.002. Epub 2018 Sep 20. Structure. 2018. PMID: 30244966 Free PMC article.
-
Structural insights into the gating mechanisms of TRPV channels.Cell Calcium. 2020 May;87:102168. doi: 10.1016/j.ceca.2020.102168. Epub 2020 Jan 24. Cell Calcium. 2020. PMID: 32004816 Free PMC article. Review.
-
TRP Channels Regulation of Rho GTPases in Brain Context and Diseases.Front Cell Dev Biol. 2020 Nov 10;8:582975. doi: 10.3389/fcell.2020.582975. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33240883 Free PMC article. Review.
References
-
- Abbott A. They said it couldn’t be done. Nature. 2002;418:268–269. - PubMed
-
- Bates-Withers C, Sah R, Clapham DE. TRPM7, the Mg(2+) inhibited channel and kinase. Advances in experimental medicine and biology. 2011;704:173–183. - PubMed
-
- Bill RM, Henderson PJ, Iwata S, Kunji ER, Michel H, Neutze R, Newstead S, Poolman B, Tate CG, Vogel H. Overcoming barriers to membrane protein structure determination. Nature biotechnology. 2011;29:335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

