Evaluation of granulated lactose as a carrier for DPI formulations 1: effect of granule size
- PMID: 24962007
- PMCID: PMC4245420
- DOI: 10.1208/s12249-014-0166-z
Evaluation of granulated lactose as a carrier for DPI formulations 1: effect of granule size
Abstract
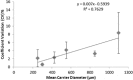
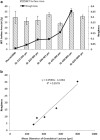
The objective of this study was to investigate the effect of large granulated lactose carrier particle systems on aerosol performance of dry powder inhaler formulations. Granulated lactose carriers with average sizes ranging from 200 to 1,000 μm were prepared and subsequently fractionated into separate narrow size powders. The fractionated granulated lactose (GL) samples were characterized in terms of size, specific surface area, surface roughness, morphology, density, flowability, and solid-state. The in vitro aerosolization performance was performed on the different size fractions of GL samples from a commercial inhaler device (Aerolizer®) with a model formulation (2% w/w salbutamol sulfate). The cascade impaction parameters employed were 60 or 90 L/min with standard (aperture size, 0.6 mm) or modified piercing holes (aperture size, 1.2 mm) of the inhaler loaded capsules. It was shown that the largest size fraction formulation (850-1000 μm) had a slight improvement in the fine particle fraction (FPF) compared to immediately preceding size fractions, explained by a smaller adhesive force between drug and carrier. Compared to commercial piercing holes, enlarged piercing holes generated a slight decreasing trend of FPF as the lactose powder sizes increased from 200-250 μm to 600-850 μm, perhaps due to the reduced detachment force by flow forces. The size, surface roughness, density, and flowability of lactose carrier as well as device design all contributed to the aerosol dispersion performance of granulated lactose-based adhesive mixtures. It was concluded that poorer or enhanced redispersion performance is not an inherent property to the significantly large size of granulated lactose carriers as previously contended.
Figures





Similar articles
-
Dry powder inhalers: mechanistic evaluation of lactose formulations containing salbutamol sulphate.Int J Pharm. 2012 Feb 28;423(2):184-94. doi: 10.1016/j.ijpharm.2011.12.018. Epub 2011 Dec 17. Int J Pharm. 2012. PMID: 22197772
-
Evaluation of Granulated Lactose as a Carrier for Dry Powder Inhaler Formulations 2: Effect of Drugs and Drug Loading.J Pharm Sci. 2017 Jan;106(1):366-376. doi: 10.1016/j.xphs.2016.09.035. J Pharm Sci. 2017. PMID: 27939234
-
Improved aerosolization performance of salbutamol sulfate formulated with lactose crystallized from binary mixtures of ethanol-acetone.J Pharm Sci. 2011 Jul;100(7):2665-84. doi: 10.1002/jps.22483. Epub 2011 Jan 25. J Pharm Sci. 2011. PMID: 21268026
-
Drug-lactose binding aspects in adhesive mixtures: controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces.Adv Drug Deliv Rev. 2012 Mar 15;64(3):275-84. doi: 10.1016/j.addr.2011.07.002. Epub 2011 Jul 18. Adv Drug Deliv Rev. 2012. PMID: 21782866 Review.
-
Recent developments in lactose blend formulations for carrier-based dry powder inhalation.Adv Drug Deliv Rev. 2022 Oct;189:114527. doi: 10.1016/j.addr.2022.114527. Epub 2022 Sep 5. Adv Drug Deliv Rev. 2022. PMID: 36070848 Review.
Cited by
-
Dry Powder Inhalation for Lung Delivery in Cystic Fibrosis.Pharmaceutics. 2023 May 13;15(5):1488. doi: 10.3390/pharmaceutics15051488. Pharmaceutics. 2023. PMID: 37242730 Free PMC article. Review.
-
Different Carriers for Use in Dry Powder Inhalers: Characteristics of Their Particles.J Aerosol Med Pulm Drug Deliv. 2024 Dec;37(6):307-327. doi: 10.1089/jamp.2023.0029. Epub 2024 Aug 9. J Aerosol Med Pulm Drug Deliv. 2024. PMID: 39120712 Review.
-
Spray-Congealing and Wet-Sieving as Alternative Processes for Engineering of Inhalation Carrier Particles: Comparison of Surface Properties, Blending and In Vitro Performance.Pharm Res. 2021 Jun;38(6):1107-1123. doi: 10.1007/s11095-021-03061-5. Epub 2021 Jun 10. Pharm Res. 2021. PMID: 34114162 Free PMC article.
-
Lactose: Characteristics, Food and Drug-Related Applications, and Its Possible Substitutions in Meeting the Needs of People with Lactose Intolerance.Foods. 2022 May 19;11(10):1486. doi: 10.3390/foods11101486. Foods. 2022. PMID: 35627056 Free PMC article. Review.
-
Optimization of Carrier-Based Dry Powder Inhaler Performance: A Review.Pharmaceutics. 2025 Jan 13;17(1):96. doi: 10.3390/pharmaceutics17010096. Pharmaceutics. 2025. PMID: 39861744 Free PMC article. Review.
References
-
- Hickey AJ. Pharmaceutical inhalation aerosol powder dispersion—an unbalancing act. Am Pharm Rev. 2003;6:106–10.
-
- Smyth HD, Hickey AJ. Carriers in drug powder delivery. Am J Drug Deliv. 2005;3:117–32. doi: 10.2165/00137696-200503020-00004. - DOI
-
- Steckel H, Muller BW. In vitro evaluation of dry powder inhalers. 2. Influence of carrier particle size and concentration on in vitro deposition. Int J Pharm. 1997;154:31–7. doi: 10.1016/S0378-5173(97)00115-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

