CSNK1α1 mediates malignant plasma cell survival
- PMID: 24962017
- PMCID: PMC4276736
- DOI: 10.1038/leu.2014.202
CSNK1α1 mediates malignant plasma cell survival
Abstract
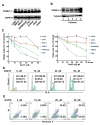
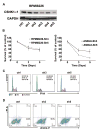
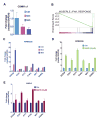
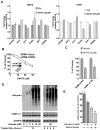
Here we report that targeting casein kinase 1-α1 (CSNK1α1) is a potential novel treatment strategy in multiple myeloma (MM) therapy distinct from proteasome inhibition. CSNK1α1 is expressed in all the tested MM cell lines and patient MM cells, and is not altered during bortezomib-triggered cytotoxicity. Inhibition of CSNK1α1 kinase activity in MM cells with targeted therapy D4476 or small hairpin RNAs triggers cell G0/G1-phase arrest, prolonged G2/M phase and apoptosis. D4476 also induced cytotoxicity in bortezomib-resistant MM cells and enhanced bortezomib-triggered cytotoxicity. CSNK1α1 signaling pathways include CDKN1B, P53 and FADD; gene signatures involved included interferon-α, tumor necrosis factor-α and LIN9. In addition, reduction of Csnk1α1 prevents cMYC/KRAS12V transformation of BaF3 cells independent of interleukin-3. Impartially, reducing Csnk1α1 prevented development of cMYC/KRAS12V-induced plasmacytomas in mice, suggesting that CSNK1α1 may be involved in MM initiation and progression. Our data suggest that targeting CSNK1α1, alone or combined with bortezomib, is a potential novel therapeutic strategy in MM. Moreover, inhibition of CSNK1α1 may prevent the progression of monoclonal gammopathy of undetermined significance to MM.
Conflict of interest statement
Conflict-of-interest disclosure: The authors have no conflicting financial interests.
Figures



References
-
- Palumbo A, Anderson K. Multiple myeloma. The New England journal of medicine. 2011 Mar 17;364(11):1046–1060. - PubMed
-
- Anderson KC. New insights into therapeutic targets in myeloma. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2011;2011:184–190. - PubMed
-
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. The oncologist. 2003;8(6):508–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

