Clinical hypothermia temperatures increase complement activation and cell destruction via the classical pathway
- PMID: 24962100
- PMCID: PMC4079622
- DOI: 10.1186/1479-5876-12-181
Clinical hypothermia temperatures increase complement activation and cell destruction via the classical pathway
Abstract
Background: Therapeutic hypothermia is a treatment modality that is increasingly used to improve clinical neurological outcomes for ischemia-reperfusion injury-mediated diseases. Antibody-initiated classical complement pathway activation has been shown to contribute to ischemia-reperfusion injury in multiple disease processes. However, how therapeutic hypothermia affects complement activation is unknown. Our goal was to measure the independent effect of temperature on complement activation, and more specifically, examine the relationship between clinical hypothermia temperatures (31-33°C), and complement activation.
Methods: Antibody-sensitized erythrocytes were used to assay complement activation at temperatures ranging from 0-41°C. Individual complement pathway components were assayed by ELISA, Western blot, and quantitative dot blot. Peptide Inhibitor of complement C1 (PIC1) was used to specifically inhibit activation of C1.
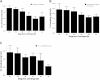
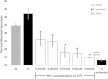
Results: Antibody-initiated complement activation resulting in eukaryotic cell lysis was increased by 2-fold at 31°C compared with 37°C. Antibody-initiated complement activation in human serum increased as temperature decreased from 37°C until dramatically decreasing at 13°C. Quantitation of individual complement components showed significantly increased activation of C4, C3, and C5 at clinical hypothermia temperatures. In contrast, C1s activation by heat-aggregated IgG decreased at therapeutic hypothermia temperatures consistent with decreased enzymatic activity at lower temperatures. However, C1q binding to antibody-coated erythrocytes increased at lower temperatures, suggesting that increased classical complement pathway activation is mediated by increased C1 binding at therapeutic hypothermia temperatures. PIC1 inhibited hypothermia-enhanced complement-mediated cell lysis at 31°C by up to 60% (P = 0.001) in a dose dependent manner.
Conclusions: In summary, therapeutic hypothermia temperatures increased antibody-initiated complement activation and eukaryotic cell destruction suggesting that the benefits of therapeutic hypothermia may be mediated via other mechanisms. Antibody-initiated complement activation has been shown to contribute to ischemia-reperfusion injury in several animal models, suggesting that for diseases with this mechanism hypothermia-enhanced complement activation may partially attenuate the benefits of therapeutic hypothermia.
Figures




Similar articles
-
Specific inhibition of the classical complement pathway with an engineered single-chain Fv to C1q globular heads decreases complement activation by apoptotic cells.Immunobiology. 2010 May;215(5):395-405. doi: 10.1016/j.imbio.2009.05.010. Epub 2009 Jul 7. Immunobiology. 2010. PMID: 19586684
-
Peptide inhibitor of complement c1, a novel suppressor of classical pathway activation: mechanistic studies and clinical potential.Front Immunol. 2014 Aug 22;5:406. doi: 10.3389/fimmu.2014.00406. eCollection 2014. Front Immunol. 2014. PMID: 25202312 Free PMC article. Review.
-
Peptide Inhibitor of Complement C1 (PIC1) Rapidly Inhibits Complement Activation after Intravascular Injection in Rats.PLoS One. 2015 Jul 21;10(7):e0132446. doi: 10.1371/journal.pone.0132446. eCollection 2015. PLoS One. 2015. PMID: 26196285 Free PMC article.
-
Activation of the classical pathway of complement by Hageman factor fragment.J Exp Med. 1981 Mar 1;153(3):665-76. doi: 10.1084/jem.153.3.665. J Exp Med. 1981. PMID: 7252410 Free PMC article.
-
Complement: activation, consequences, and control.Am J Med Technol. 1982 Sep;48(9):735-42. Am J Med Technol. 1982. PMID: 6215861 Review.
Cited by
-
Mild hypothermia inhibits systemic and cerebral complement activation in a swine model of cardiac arrest.J Cereb Blood Flow Metab. 2015 Aug;35(8):1289-95. doi: 10.1038/jcbfm.2015.41. Epub 2015 Mar 11. J Cereb Blood Flow Metab. 2015. PMID: 25757755 Free PMC article.
-
Classical complement pathway inhibition reduces brain damage in a hypoxic ischemic encephalopathy animal model.PLoS One. 2021 Sep 30;16(9):e0257960. doi: 10.1371/journal.pone.0257960. eCollection 2021. PLoS One. 2021. PMID: 34591905 Free PMC article.
-
The Atlantic salmon's stress- and immune-related transcriptional responses to moderate hypoxia, an incremental temperature increase, and these challenges combined.G3 (Bethesda). 2021 Jul 14;11(7):jkab102. doi: 10.1093/g3journal/jkab102. G3 (Bethesda). 2021. PMID: 34015123 Free PMC article.
-
Therapeutic hypothermia modulates complement factor C3a and C5a levels in a rat model of hypoxic ischemic encephalopathy.Pediatr Res. 2017 Apr;81(4):654-662. doi: 10.1038/pr.2016.271. Epub 2016 Dec 21. Pediatr Res. 2017. PMID: 28002390
-
Simulation of the trimeric globular head of C1q reveals temperature-sensitive network: implications for inflammation.J Mol Model. 2025 Aug 13;31(9):247. doi: 10.1007/s00894-025-06464-y. J Mol Model. 2025. PMID: 40801960 Free PMC article.
References
-
- Group HCAS. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. - PubMed
-
- BT Foundation, Surgeons AAoN, Surgeons CoN. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

