Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer
- PMID: 24962213
- PMCID: PMC4147322
- DOI: 10.18632/oncotarget.2001
Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer
Abstract
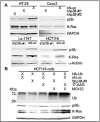
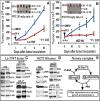
A cancer phenotype is driven by several proteins and targeting a cluster of functionally interdependent molecules should be more effective for therapeutic intervention. This is specifically important for Ras-dependent cancer, as mutated (MT) Ras is non-druggable and targeting its interaction with effectors may be essential for therapeutic intervention. Here, we report that a protein-complex activated by the Ras effector p38γ MAPK is a novel therapeutic target for K-Ras-dependent colon cancer. Unbiased proteomic screening and immune-precipitation analyses identified p38γ interaction with heat shock protein 90 (Hsp90) and K-Ras in K-Ras MT, but not wild-type (WT), colon cancer cells, indicating a role of this complex in Ras-dependent growth. Further experiments showed that this complex requires p38γ and Hsp90 activity to maintain MT, but not WT, K-Ras protein expression. Additional studies demonstrated that this complex is activated by p38γ-induced Hsp90 phosphorylation at S595, which is important for MT K-Ras stability and for K-Ras dependent growth. Of most important, pharmacologically inhibition of Hsp90 or p38γ activity disrupts the complex, decreases K-Ras expression, and selectively inhibits the growth of K-Ras MT colon cancer in vitro and in vivo. These results demonstrated that the p38γ-activated ternary complex is a novel therapeutic target for K-Ras-dependent colon cancer.
Figures
Similar articles
-
PTPH1 dephosphorylates and cooperates with p38gamma MAPK to increase ras oncogenesis through PDZ-mediated interaction.Cancer Res. 2010 Apr 1;70(7):2901-10. doi: 10.1158/0008-5472.CAN-09-3229. Epub 2010 Mar 23. Cancer Res. 2010. PMID: 20332238 Free PMC article.
-
Essential role of p38gamma in K-Ras transformation independent of phosphorylation.J Biol Chem. 2005 Jun 24;280(25):23910-7. doi: 10.1074/jbc.M500699200. Epub 2005 Apr 25. J Biol Chem. 2005. PMID: 15851477 Free PMC article.
-
p38γ Mitogen-activated protein kinase signals through phosphorylating its phosphatase PTPH1 in regulating ras protein oncogenesis and stress response.J Biol Chem. 2012 Aug 10;287(33):27895-905. doi: 10.1074/jbc.M111.335794. Epub 2012 Jun 22. J Biol Chem. 2012. PMID: 22730326 Free PMC article.
-
The Role of Wild-Type RAS in Oncogenic RAS Transformation.Genes (Basel). 2021 Apr 28;12(5):662. doi: 10.3390/genes12050662. Genes (Basel). 2021. PMID: 33924994 Free PMC article. Review.
-
The RAS-Effector Interaction as a Drug Target.Cancer Res. 2017 Jan 15;77(2):221-226. doi: 10.1158/0008-5472.CAN-16-0938. Epub 2017 Jan 6. Cancer Res. 2017. PMID: 28062402 Free PMC article. Review.
Cited by
-
p38γ MAPK is required for inflammation-associated colon tumorigenesis.Oncogene. 2016 Feb 25;35(8):1039-48. doi: 10.1038/onc.2015.158. Epub 2015 May 11. Oncogene. 2016. PMID: 25961922
-
p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer-A Comparative Study with Humans.Vaccines (Basel). 2020 Dec 2;8(4):720. doi: 10.3390/vaccines8040720. Vaccines (Basel). 2020. PMID: 33276422 Free PMC article.
-
Targeting an oncogenic kinase/phosphatase signaling network for cancer therapy.Acta Pharm Sin B. 2018 Jul;8(4):511-517. doi: 10.1016/j.apsb.2018.05.007. Epub 2018 May 22. Acta Pharm Sin B. 2018. PMID: 30109176 Free PMC article. Review.
-
Sensitivity of Oncogenic KRAS-Expressing Cells to CDK9 Inhibition.SLAS Discov. 2021 Aug;26(7):922-932. doi: 10.1177/24725552211008853. Epub 2021 Apr 24. SLAS Discov. 2021. PMID: 33896272 Free PMC article.
-
p38γ MAPK Is a Therapeutic Target for Triple-Negative Breast Cancer by Stimulation of Cancer Stem-Like Cell Expansion.Stem Cells. 2015 Sep;33(9):2738-47. doi: 10.1002/stem.2068. Epub 2015 Jun 23. Stem Cells. 2015. PMID: 26077647 Free PMC article.
References
-
- Downward J. Targeting Ras signalling pathways in cancer therapy. Nature Rev Cancer. 2003;3:11–22. - PubMed
-
- Malumbres M, Barbacid M. Ras oncogenes: the first 30 years. Nature Rev Cancer. 2003;3:7–13. - PubMed
-
- Tanaka M, Omura K, Watanabe Y, Oda Y, Nakanishi I. Prognostic factors of colorectal cancer: K-ras mutation, overexpression of the p53 protein and cell proliferative activity. J Surg Oncol. 1994;57:57–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

