Targeting the wee1 kinase for treatment of pediatric Down syndrome acute myeloid leukemia
- PMID: 24962331
- PMCID: PMC4199830
- DOI: 10.1002/pbc.25081
Targeting the wee1 kinase for treatment of pediatric Down syndrome acute myeloid leukemia
Abstract
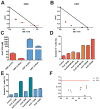
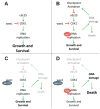
Background: Most Down syndrome children with acute myeloid leukemia (DS-AML) have an overall excellent prognosis, however, patients who suffer an induction failure or relapse, have an extremely poor prognosis. Hence, new therapies need to be developed for this subgroup of DS-AML patients. One new therapeutic approach is preventing cell cycle checkpoint activation by inhibiting the upstream kinase wee1 with the first-in-class inhibitor MK-1775 in combination with the standard genotoxic agent cytarabine (AraC).
Procedure: Using the clinically relevant DS-AML cell lines CMK and CMY, as well as ex vivo primary DS-AML patient samples, the ability of MK-1775 to enhance the cytotoxicity of AraC was investigated with MTT assays. The mechanism by which MK-1775 enhanced AraC cytotoxicity was investigated in the cell lines using Western blots to probe CDK1 and H2AX phosphorylation and flow cytometry to determine apoptosis, cell cycle arrest, DNA damage, and aberrant mitotic entry.
Results: MK-1775 alone had modest single-agent activity, however, MK-1775 was able to synergize with AraC in causing proliferation arrest in both cell lines and primary patient samples, and enhance AraC-induced apoptosis. MK-1775 was able to decrease inhibitory CDK1(Y15) phosphorylation at the relatively low concentration of 100 nM after only 4 hours. Furthermore, it was able to enhance DNA damage induced by AraC and partially abrogate cell cycle arrest. Importantly, the DNA damage enhancement appeared in early S-phase.
Conclusions: MK-1775 is able to enhance the cytotoxicity of AraC in DS-AML cells and presents a promising new treatment approach for DS-AML.
Keywords: AraC; DNA damage; Down syndrome; MK-1775; wee1.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Nothing to declare.
Figures




Similar articles
-
CHK1 plays a critical role in the anti-leukemic activity of the wee1 inhibitor MK-1775 in acute myeloid leukemia cells.J Hematol Oncol. 2014 Aug 1;7:53. doi: 10.1186/s13045-014-0053-9. J Hematol Oncol. 2014. PMID: 25084614 Free PMC article.
-
Synergistic anti-leukemic interactions between panobinostat and MK-1775 in acute myeloid leukemia ex vivo.Cancer Biol Ther. 2015;16(12):1784-93. doi: 10.1080/15384047.2015.1095406. Cancer Biol Ther. 2015. PMID: 26529495 Free PMC article.
-
Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents.Mol Cancer Ther. 2009 Nov;8(11):2992-3000. doi: 10.1158/1535-7163.MCT-09-0463. Epub 2009 Nov 3. Mol Cancer Ther. 2009. PMID: 19887545
-
Wee1 kinase as a target for cancer therapy.Cell Cycle. 2013 Oct 1;12(19):3159-64. doi: 10.4161/cc.26062. Epub 2013 Aug 26. Cell Cycle. 2013. PMID: 24013427 Free PMC article. Review.
-
Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents.Curr Clin Pharmacol. 2010 Aug;5(3):186-91. doi: 10.2174/157488410791498824. Curr Clin Pharmacol. 2010. PMID: 20406171 Review.
Cited by
-
Synergistic anti-leukemic interactions between ABT-199 and panobinostat in acute myeloid leukemia ex vivo.Am J Transl Res. 2016 Sep 15;8(9):3893-3902. eCollection 2016. Am J Transl Res. 2016. PMID: 27725868 Free PMC article.
-
Rational Combinations of Targeted Agents in AML.J Clin Med. 2015 Apr 10;4(4):634-664. doi: 10.3390/jcm4040634. eCollection 2015 Apr. J Clin Med. 2015. PMID: 26113989 Free PMC article. Review.
-
A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target.J Hematol Oncol. 2020 Sep 21;13(1):126. doi: 10.1186/s13045-020-00959-2. J Hematol Oncol. 2020. PMID: 32958072 Free PMC article. Review.
-
Prognosis and management of acute myeloid leukemia in patients with Down syndrome.Expert Rev Hematol. 2014 Dec;7(6):831-40. doi: 10.1586/17474086.2014.959923. Epub 2014 Sep 18. Expert Rev Hematol. 2014. PMID: 25231553 Free PMC article. Review.
-
RAS mutations drive proliferative chronic myelomonocytic leukemia via a KMT2A-PLK1 axis.Nat Commun. 2021 May 18;12(1):2901. doi: 10.1038/s41467-021-23186-w. Nat Commun. 2021. PMID: 34006870 Free PMC article.
References
-
- Ge Y, Jensen TL, Stout ML, et al. The role of cytidine deaminase and GATA1 mutations in the increased cytosine arabinoside sensitivity of Down syndrome myeloblasts and leukemia cell lines. Cancer Res. 2004;64:728–735. - PubMed
-
- Ge Y, Stout ML, Tatman DA, et al. GATA1, cytidine deaminase, and the high cure rate of Down syndrome children with acute megakaryocytic leukemia. J Natl Cancer Inst. 2005;97:226–231. - PubMed
-
- Taub JW, Huang X, Ge Y, et al. Cystathionine-beta-synthase cDNA transfection alters the sensitivity and metabolism of 1-beta-d-arabinofuranosylcytosine in CCRF-CEM leukemia cells in vitro and in vivo: A model of leukemia in Down syndrome. Cancer Res. 2000;60:6421–6426. - PubMed
-
- Taub JW, Matherly LH, Stout ML, et al. Enhanced metabolism of 1-beta-d-arabinofuranosylcytosine in Down syndrome cells: A contributing factor to the superior event free survival of Down syndrome children with acute myeloid leukemia. Blood. 1996;87:3395–3403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

