Inhibition of cancer derived cell lines proliferation by synthesized hydroxylated stilbenes and new ferrocenyl-stilbene analogs. Comparison with resveratrol
- PMID: 24962390
- PMCID: PMC6271691
- DOI: 10.3390/molecules19067850
Inhibition of cancer derived cell lines proliferation by synthesized hydroxylated stilbenes and new ferrocenyl-stilbene analogs. Comparison with resveratrol
Abstract
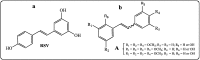
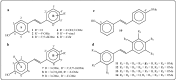
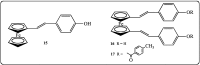
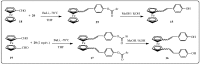
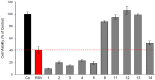
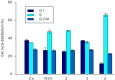
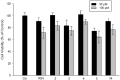
Further advances in understanding the mechanism of action of resveratrol and its application require new analogs to identify the structural determinants for the cell proliferation inhibition potency. Therefore, we synthesized new trans-resveratrol derivatives by using the Wittig and Heck methods, thus modifying the hydroxylation and methoxylation patterns of the parent molecule. Moreover, we also synthesized new ferrocenylstilbene analogs by using an original protective group in the Wittig procedure. By performing cell proliferation assays we observed that the resveratrol derivatives show inhibition on the human colorectal tumor SW480 cell line. On the other hand, cell viability/cytotoxicity assays showed a weaker effects on the human hepatoblastoma HepG2 cell line. Importantly, the lack of effect on non-tumor cells (IEC18 intestinal epithelium cells) demonstrates the selectivity of these molecules for cancer cells. Here, we show that the numbers and positions of hydroxy and methoxy groups are crucial for the inhibition efficacy. In addition, the presence of at least one phenolic group is essential for the antitumoral activity. Moreover, in the series of ferrocenylstilbene analogs, the presence of a hidden phenolic function allows for a better solubilization in the cellular environment and significantly increases the antitumoral activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
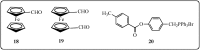

Similar articles
-
Structural determinants of resveratrol for cell proliferation inhibition potency: experimental and docking studies of new analogs.Eur J Med Chem. 2010 Jul;45(7):2972-80. doi: 10.1016/j.ejmech.2010.03.024. Epub 2010 Mar 25. Eur J Med Chem. 2010. PMID: 20395019
-
Synthesis and Evaluation of 2-Naphthaleno trans-Stilbenes and Cyanostilbenes as Anticancer Agents.Anticancer Agents Med Chem. 2018;18(4):556-564. doi: 10.2174/1871521409666170412115703. Anticancer Agents Med Chem. 2018. PMID: 28403783 Free PMC article.
-
Differential effects of resveratrol and its naturally occurring methylether analogs on cell cycle and apoptosis in human androgen-responsive LNCaP cancer cells.Mol Nutr Food Res. 2010 Mar;54(3):335-44. doi: 10.1002/mnfr.200900143. Mol Nutr Food Res. 2010. PMID: 20077416
-
Effects of hydroxylated resveratrol analogs on oxidative stress and cancer cells death in human acute T cell leukemia cell line: prooxidative potential of hydroxylated resveratrol analogs.Chem Biol Interact. 2014 Feb 25;209:96-110. doi: 10.1016/j.cbi.2013.12.009. Epub 2014 Jan 4. Chem Biol Interact. 2014. PMID: 24398169
-
Resveratrol and other Stilbenes: Effects on Dysregulated Gene Expression in Cancers and Novel Delivery Systems.Anticancer Agents Med Chem. 2021;21(5):567-574. doi: 10.2174/1871520620666200705220722. Anticancer Agents Med Chem. 2021. PMID: 32628597 Review.
Cited by
-
Stilbene Glycosides in Pinus cembra L. Bark: Isolation, Characterization, and Assessment of Antioxidant Potential and Antitumor Activity on HeLa Cells.Plants (Basel). 2025 May 14;14(10):1459. doi: 10.3390/plants14101459. Plants (Basel). 2025. PMID: 40431024 Free PMC article.
-
Elaboration of Trans-Resveratrol Derivative-Loaded Superparamagnetic Iron Oxide Nanoparticles for Glioma Treatment.Nanomaterials (Basel). 2019 Feb 18;9(2):287. doi: 10.3390/nano9020287. Nanomaterials (Basel). 2019. PMID: 30781702 Free PMC article.
-
New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells.Molecules. 2021 May 1;26(9):2661. doi: 10.3390/molecules26092661. Molecules. 2021. PMID: 34062841 Free PMC article.
-
Aza- and Azo-Stilbenes: Bio-Isosteric Analogs of Resveratrol.Molecules. 2020 Jan 30;25(3):605. doi: 10.3390/molecules25030605. Molecules. 2020. PMID: 32019195 Free PMC article. Review.
-
Resveratrol-Induced Xenophagy Promotes Intracellular Bacteria Clearance in Intestinal Epithelial Cells and Macrophages.Front Immunol. 2019 Jan 14;9:3149. doi: 10.3389/fimmu.2018.03149. eCollection 2018. Front Immunol. 2019. PMID: 30693000 Free PMC article.
References
-
- Watt J.M., Breyer-Brandwijk M.G. Medicinal and poisonous plants. Nature. 1962;196:609–610.
-
- Langcake P., Pryce R. The production of Resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant. Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

