Catalytic behavior of lipase immobilized onto congo red and PEG-decorated particles
- PMID: 24962395
- PMCID: PMC6271667
- DOI: 10.3390/molecules19068610
Catalytic behavior of lipase immobilized onto congo red and PEG-decorated particles
Abstract
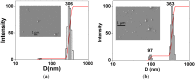
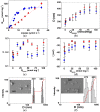
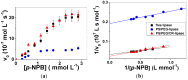
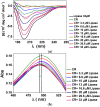
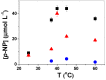
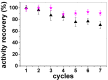
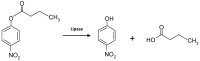
Poly(ethylene glycol) (PEG)-decorated polystyrene (PS) nanoparticles with mean hydrodynamic diameter (D) and zeta-potential (ζ) of (286 ± 15) nm and (-50 ± 5) mV, respectively, were modified by the adsorption of Congo red (CR). The PS/PEG/CR particles presented D and ζ values of (290 ± 19) nm and (-36 ± 5) mV, respectively. The adsorption of lipase onto PS/PEG or PS/PEG/CR particles at (24 ± 1) °C and pH 7 changed the mean D value to (380 ± 20) and (405 ± 11) nm, respectively, and ζ value to (-32 ± 4) mV and (-25 ± 2) mV, respectively. The kinetic parameters of the hydrolysis of p-nitrophenyl butyrate were determined for free lipase, lipase immobilized onto PS/PEG and PS/PEG/CR particles. Lipase on PS/PEG/CR presented the largest Michaelis-Menten constant (KM), but also the highest Vmax and kcat values. Moreover, it could be recycled seven times, losing a maximum 10% or 30% of the original enzymatic activity at 40 °C or 25 °C, respectively. Although lipases immobilized onto PS/PEG particles presented the smallest KM values, the reactions were comparatively the slowest and recycling was not possible. Hydrolysis reactions performed in the temperature range of 25 °C to 60 °C with free lipases and lipases immobilized onto PS/PEG/CR particles presented an optimal temperature at 40 °C. At 60 °C free lipases and lipases immobilized onto PS/PEG/CR presented ~80% and ~50% of the activity measured at 40 °C, indicating good thermal stability. Bioconjugation effects between CR and lipase were evidenced by circular dichroism spectroscopy and spectrophotometry. CR molecules mediate the open state conformation of the lipase lid and favor the substrate approaching.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
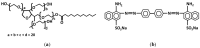
References
-
- Di Cosimo R., McAuliffe J., Poulose A.J., Bohlmann G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013;42:6437–6474. - PubMed
-
- Kapoor M., Gupta M.N. Lipase promiscuity and its biochemical applications. Proc. Biochem. 2012;47:555–569. doi: 10.1016/j.procbio.2012.01.011. - DOI
-
- Carrasco-Lopez C., Godoy C., de las Rivas B., Fernandez-Lorente G., Palomo J.M., Guisan J.M. Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements. J. Biol. Chem. 2009;284:4365–4372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

