CRL4Cdt2 E3 ubiquitin ligase and proliferating cell nuclear antigen (PCNA) cooperate to degrade thymine DNA glycosylase in S phase
- PMID: 24962565
- PMCID: PMC4132804
- DOI: 10.1074/jbc.M114.574210
CRL4Cdt2 E3 ubiquitin ligase and proliferating cell nuclear antigen (PCNA) cooperate to degrade thymine DNA glycosylase in S phase
Abstract
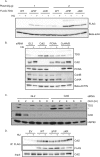
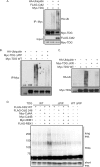
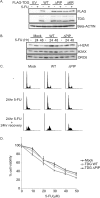
Thymine DNA glycosylase (TDG) is an essential enzyme playing multiple roles in base excision repair, transcription regulation, and DNA demethylation. TDG mediates the cytotoxicity of the anti-cancer chemotherapeutic drug 5-fluorouracil (5-FU) by prolonging S phase, generating DNA strand breaks, and inducing DNA damage signaling. During S phase of the cell cycle, TDG is degraded via the proteasomal pathway. Here we show that CRL4(Cdt2) E3 ubiquitin ligase promotes ubiquitination and proteasomal degradation of TDG in S phase in a reaction that is dependent on the interaction of TDG with proliferating cell nuclear antigen (PCNA). siRNA-mediated depletion of PCNA or components of CRL4(Cdt2), specifically cullin4A/B or substrate adaptor Cdt2, stabilizes TDG in human cells. Mutations in the PCNA-interacting peptide (PIP) motif of TDG that disrupt the interaction of TDG with PCNA or change critical basic residues essential for the action of the PIP degron prevent the ubiquitination and degradation of TDG. Thus physical interaction of TDG with PCNA through the PIP degron is required for targeting TDG to the CRL4(Cdt2) E3 ubiquitin ligase complex. Compared with forced expression of wild type TDG, CRL4(Cdt2)- resistant TDG (ΔPIP) slows cell proliferation and slightly increases the toxicity of 5-FU. Thus, CRL4(Cdt2)-dependent degradation of TDG occurs in S phase because of the requirement for TDG to interact with chromatin-loaded PCNA, and this degradation is important for preventing toxicity from excess TDG.
Keywords: DNA Damage Response; E3 Ubiquitin Ligase; Proliferating Cell Nuclear Antigen (PCNA); Protein Degradation; Thymine-DNA Glycosylase (TDG); Ubiquitin.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Thymine DNA glycosylase is a CRL4Cdt2 substrate.J Biol Chem. 2014 Aug 15;289(33):23043-23055. doi: 10.1074/jbc.M114.574194. Epub 2014 Jun 19. J Biol Chem. 2014. PMID: 24947512 Free PMC article.
-
CDK1-dependent inhibition of the E3 ubiquitin ligase CRL4CDT2 ensures robust transition from S Phase to Mitosis.J Biol Chem. 2015 Jan 2;290(1):556-67. doi: 10.1074/jbc.M114.614701. Epub 2014 Nov 19. J Biol Chem. 2015. PMID: 25411249 Free PMC article.
-
Proliferating cell nuclear antigen interacts with the CRL4 ubiquitin ligase subunit CDT2 in DNA synthesis-induced degradation of CDT1.J Biol Chem. 2018 Dec 7;293(49):18879-18889. doi: 10.1074/jbc.RA118.003049. Epub 2018 Oct 9. J Biol Chem. 2018. PMID: 30301766 Free PMC article.
-
CRL4Cdt2 Ubiquitin Ligase, A Genome Caretaker Controlled by Cdt2 Binding to PCNA and DNA.Genes (Basel). 2022 Jan 29;13(2):266. doi: 10.3390/genes13020266. Genes (Basel). 2022. PMID: 35205311 Free PMC article. Review.
-
Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase.Genes Dev. 2011 Aug 1;25(15):1568-82. doi: 10.1101/gad.2068611. Genes Dev. 2011. PMID: 21828267 Free PMC article. Review.
Cited by
-
Ionic strength modulates excision of uracil by SMUG1 from nucleosome core particles.DNA Repair (Amst). 2023 May;125:103482. doi: 10.1016/j.dnarep.2023.103482. Epub 2023 Mar 12. DNA Repair (Amst). 2023. PMID: 36931160 Free PMC article.
-
Defining the Role of Nucleotide Flipping in Enzyme Specificity Using 19F NMR.J Am Chem Soc. 2019 Mar 27;141(12):4952-4962. doi: 10.1021/jacs.9b00146. Epub 2019 Mar 14. J Am Chem Soc. 2019. PMID: 30841696 Free PMC article.
-
Thymine DNA glycosylase is a CRL4Cdt2 substrate.J Biol Chem. 2014 Aug 15;289(33):23043-23055. doi: 10.1074/jbc.M114.574194. Epub 2014 Jun 19. J Biol Chem. 2014. PMID: 24947512 Free PMC article.
-
CUL4-Based Ubiquitin Ligases in Chromatin Regulation: An Evolutionary Perspective.Cells. 2025 Jan 7;14(2):63. doi: 10.3390/cells14020063. Cells. 2025. PMID: 39851492 Free PMC article. Review.
-
Characterizing Requirements for Small Ubiquitin-like Modifier (SUMO) Modification and Binding on Base Excision Repair Activity of Thymine-DNA Glycosylase in Vivo.J Biol Chem. 2016 Apr 22;291(17):9014-24. doi: 10.1074/jbc.M115.706325. Epub 2016 Feb 25. J Biol Chem. 2016. PMID: 26917720 Free PMC article.
References
-
- Hendrich B., Hardeland U., Ng H. H., Jiricny J., Bird A. (1999) The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature 401, 301–304 - PubMed
-
- Haushalter K. A., Todd Stukenberg M. W., Kirschner M. W., Verdine G. L. (1999) Identification of a new uracil-DNA glycosylase family by expression cloning using synthetic inhibitors. Curr. Biol. 9, 174–185 - PubMed
-
- Hardeland U., Bentele M., Lettieri T., Steinacher R., Jiricny J., Schar P. (2001) Thymine DNA glycosylase. Prog. Nucleic Acids Res. Mol. Biol. 68, 235–253 - PubMed
-
- Slupphaug G., Eftedal I., Kavli B., Bharati S., Helle N. M., Haug T., Levine D. W., Krokan H. E. (1995) Properties of a recombinant human uracil-DNA glycosylase from the UNG gene and evidence that UNG encodes the major uracil-DNA glycosylase. Biochemistry 34, 128–138 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

