Forces and dynamics of glucose and inhibitor binding to sodium glucose co-transporter SGLT1 studied by single molecule force spectroscopy
- PMID: 24962566
- PMCID: PMC4118126
- DOI: 10.1074/jbc.M113.529875
Forces and dynamics of glucose and inhibitor binding to sodium glucose co-transporter SGLT1 studied by single molecule force spectroscopy
Abstract
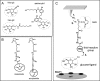
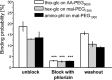
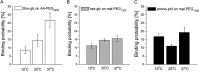
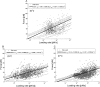
Single molecule force spectroscopy was employed to investigate the dynamics of the sodium glucose co-transporter (SGLT1) upon substrate and inhibitor binding on the single molecule level. CHO cells stably expressing rbSGLT1 were probed by using atomic force microscopy tips carrying either thioglucose, 2'-aminoethyl β-d-glucopyranoside, or aminophlorizin. Poly(ethylene glycol) (PEG) chains of different length and varying end groups were used as tether. Experiments were performed at 10, 25 and 37 °C to address different conformational states of SGLT1. Unbinding forces between ligands and SGLT1 were recorded at different loading rates by changing the retraction velocity, yielding binding probability, width of energy barrier of the binding pocket, and the kinetic off rate constant of the binding reaction. With increasing temperature, width of energy barrier and average life time increased for the interaction of SGLT1 with thioglucose (coupled via acrylamide to a long PEG) but decreased for aminophlorizin binding. The former indicates that in the membrane-bound SGLT1 the pathway to sugar translocation involves several steps with different temperature sensitivity. The latter suggests that also the aglucon binding sites for transport inhibitors have specific, temperature-sensitive conformations.
Keywords: Atomic Force Microscopy (AFM); Glucose Translocation Pathway; Glucose Transport; Kinetics; Ligand-binding Protein; Off Rate Constant; Phlorizin Binding; SGLT1; Spectroscopy; Width of Energy Barrier.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Ligands on the string: single-molecule AFM studies on the interaction of antibodies and substrates with the Na+-glucose co-transporter SGLT1 in living cells.J Cell Sci. 2006 Jul 15;119(Pt 14):2960-7. doi: 10.1242/jcs.03035. Epub 2006 Jun 20. J Cell Sci. 2006. PMID: 16787940
-
Substrate specificity of sugar transport by rabbit SGLT1: single-molecule atomic force microscopy versus transport studies.Biochemistry. 2007 Mar 13;46(10):2797-804. doi: 10.1021/bi061917z. Epub 2007 Feb 16. Biochemistry. 2007. PMID: 17302432
-
Three surface subdomains form the vestibule of the Na+/glucose cotransporter SGLT1.J Biol Chem. 2007 Aug 31;282(35):25222-30. doi: 10.1074/jbc.M704190200. Epub 2007 Jul 6. J Biol Chem. 2007. PMID: 17616521
-
Development of SGLT1 and SGLT2 inhibitors.Diabetologia. 2018 Oct;61(10):2079-2086. doi: 10.1007/s00125-018-4654-7. Epub 2018 Aug 22. Diabetologia. 2018. PMID: 30132033 Free PMC article. Review.
-
The Na+-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer.Pharmacol Ther. 2017 Feb;170:148-165. doi: 10.1016/j.pharmthera.2016.10.017. Epub 2016 Oct 20. Pharmacol Ther. 2017. PMID: 27773781 Review.
Cited by
-
Allosterically Linked Binding Sites in Serotonin Transporter Revealed by Single Molecule Force Spectroscopy.Front Mol Biosci. 2020 Jun 3;7:99. doi: 10.3389/fmolb.2020.00099. eCollection 2020. Front Mol Biosci. 2020. PMID: 32656227 Free PMC article. Review.
-
Plant glucose transporter structure and function.Pflugers Arch. 2020 Sep;472(9):1111-1128. doi: 10.1007/s00424-020-02449-3. Epub 2020 Aug 26. Pflugers Arch. 2020. PMID: 32845347 Free PMC article. Review.
-
Amino acid polymorphisms in the fibronectin-binding repeats of fibronectin-binding protein A affect bond strength and fibronectin conformation.J Biol Chem. 2017 May 26;292(21):8797-8810. doi: 10.1074/jbc.M117.786012. Epub 2017 Apr 11. J Biol Chem. 2017. PMID: 28400484 Free PMC article.
-
Atomic force microscopy as a tool to evaluate the risk of cardiovascular diseases in patients.Nat Nanotechnol. 2016 Aug;11(8):687-92. doi: 10.1038/nnano.2016.52. Epub 2016 May 16. Nat Nanotechnol. 2016. PMID: 27183056
-
Characterization of the specific interaction between the DNA aptamer sgc8c and protein tyrosine kinase-7 receptors at the surface of T-cells by biosensing AFM.Anal Bioanal Chem. 2017 Apr;409(11):2767-2776. doi: 10.1007/s00216-017-0238-5. Epub 2017 Feb 22. Anal Bioanal Chem. 2017. PMID: 28229174 Free PMC article.
References
-
- Crane R. K. (1977) The gradient hypothesis and other models of carrier-mediated active transport. Rev. Physiol. Biochem. Pharmacol. 78, 99–159 - PubMed
-
- Wright E. M., Loo D. D., Hirayama B. A. (2011) Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794 - PubMed
-
- Wright E. M. (1998) I. Glucose galactose malabsorption. Am. J. Physiol. 275, G879–G882 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

