SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability
- PMID: 24962569
- PMCID: PMC4132816
- DOI: 10.1074/jbc.M114.567107
SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability
Abstract
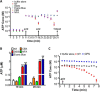
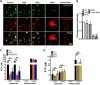
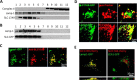
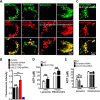
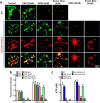
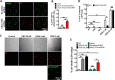
Lysosomes contain abundant ATP, which is released through lysosomal exocytosis following exposure to various stimuli. However, the molecular mechanisms underlying lysosomal ATP accumulation remain unknown. The vesicular nucleotide transporter, also known as solute carrier family 17 member 9 (SLC17A9), has been shown to function in ATP transport across secretory vesicles/granules membrane in adrenal chromaffin cells, T cells, and pancreatic cells. Here, using mammalian cell lines, we report that SLC17A9 is highly enriched in lysosomes and functions as an ATP transporter in those organelles. SLC17A9 deficiency reduced lysosome ATP accumulation and compromised lysosome function, resulting in cell death. Our data suggest that SLC17A9 activity mediates lysosomal ATP accumulation and plays an important role in lysosomal physiology and cell viability.
Keywords: ATP; Cell Death; Lysosome; Transporter; Vacuolar ATPase; Vesicular Nucleotide Transporter.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Fredriksson R., Nordström K. J., Stephansson O., Hägglund M. G., Schiöth H. B. (2008) The solute carrier (SLC) complement of the human genome: phylogenetic classification reveals four major families. FEBS Lett. 582, 3811–3816 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

