Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells
- PMID: 24962570
- PMCID: PMC4139220
- DOI: 10.1074/jbc.M114.568790
Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells
Abstract
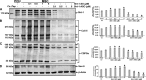
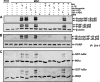
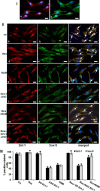
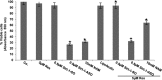
Sirtuin-1 (SIRT1), NAD(+)-dependent deacetylase, has been linked to anabolic effects in cartilage, although the mechanisms of SIRT1 signaling during differentiation of mesenchymal stem cells (MSCs) to chondrocytes are poorly understood. Therefore, we investigated the role of SIRT1-mediated signaling during chondrogenic differentiation of MSCs in vitro. High density and alginate cultures of MSCs were treated with chondrogenic induction medium with/without the SIRT1 inhibitor nicotinamide, antisense oligonucleotides against SIRT1 (SIRT1-ASO), IL-1β, and/or resveratrol. Transient transfection of MSCs with SIRT1-antisense oligonucleotides, nicotinamide, and IL-1β inhibited chondrogenesis-induced down-regulation of cartilage-specific proteins, cartilage-specific transcription factor Sox9, and enhanced NF-κB-regulated gene products involved in the inflammatory and degradative processes in cartilage (MMP-9, COX-2, and caspase-3), and NF-κB phosphorylation, acetylation, and activation of IκBα kinase. In contrast, the SIRT1 activator resveratrol or BMS-345541 (inhibitor of IKK) inhibited IL-1β- and NAM-induced suppression of cartilage-specific proteins, Sox9, and up-regulation of NF-κB-regulated gene products. Moreover, SIRT1 was found to interact directly with NF-κB and resveratrol-suppressed IL-1β and NAM but not SIRT1-ASO-induced NF-κB phosphorylation, acetylation, and activation of IκBα kinase. Knockdown of SIRT1 by mRNA abolished the inhibitory effects of resveratrol on inflammatory and apoptotic signaling and Sox9 expression, suggesting the essential role of this enzyme. Finally, the modulatory effects of resveratrol were found to be mediated at least in part by the association between SIRT1 and Sox9. These results indicate for the first time that SIRT1 supports chondrogenic development of MSCs at least in part through inhibition/deacetylation of NF-κB and activation of Sox9.
Keywords: Chondrogenesis; Inflammation; Mesenchymal Stem Cells (MSCs); NF-κB; Resveratrol; Rheumatoid Arthritis; SIRT1-ASO; SOX9; Sirtuin 1 (SIRT1).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 - PubMed
-
- Csaki C., Matis U., Mobasheri A., Ye H., Shakibaei M. (2007) Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem. Cell Biol. 128, 507–520 - PubMed
-
- Jaiswal N., Haynesworth S. E., Caplan A. I., Bruder S. P. (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 64, 295–312 - PubMed
-
- Csaki C., Schneider P. R., Shakibaei M. (2008) Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann. Anat. 190, 395–412 - PubMed
-
- Lee E. H., Hui J. H. (2006) The potential of stem cells in orthopaedic surgery. J. Bone Joint Surg. Br. 88, 841–851 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

