Specific targeting of a naturally presented osteosarcoma antigen, papillomavirus binding factor peptide, using an artificial monoclonal antibody
- PMID: 24962571
- PMCID: PMC4139219
- DOI: 10.1074/jbc.M114.568725
Specific targeting of a naturally presented osteosarcoma antigen, papillomavirus binding factor peptide, using an artificial monoclonal antibody
Abstract
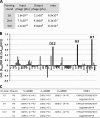
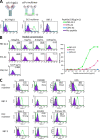
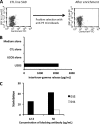
Osteosarcoma is a rare but highly malignant tumor occurring most frequently in adolescents. The prognosis of non-responders to chemotherapy is still poor, and new treatment modalities are needed. To develop peptide-based immunotherapy, we previously identified autologous cytotoxic T lymphocyte-defined osteosarcoma antigen papillomavirus binding factor (PBF) in the context of HLA-B55 and the cytotoxic T lymphocyte epitope (PBF A2.2) presented by HLA-A2. PBF and HLA class I are expressed in ∼90 and 70% of various sarcomas, respectively. However, the expression status of peptide PBF A2.2 presented by HLA-A2 on osteosarcoma cells has remained unknown because it is difficult to generate a specific probe that reacts with the HLA·peptide complex. For detection and qualification of the HLA-A*02:01·PBF A2.2 peptide complex on osteosarcoma cells, we tried to isolate a single chain variable fragment (scFv) antibody directed to the HLA-*A0201·PBF A2.2 complex using a naïve scFv phage display library. As a result, scFv clone D12 with high affinity (KD = 1.53 × 10(-9) M) was isolated. D12 could react with PBF A2.2 peptide-pulsed T2 cells and HLA-A2+PBF+ osteosarcoma cell lines and simultaneously demonstrated that the HLA·peptide complex was expressed on osteosarcoma cells. In conclusion, scFv clone D12 might be useful to select candidate patients for PBF A2.2 peptide-based immunotherapy and develop antibody-based immunotherapy.
Keywords: Antibody Engineering; Antigen Presentation; HLA-A2; HLA/Peptide Complex; Major Histocompatibility Complex (MHC); PBF; Phage Display; T Cell Receptor (TCR); scFv.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Development of a T-cell receptor multimer with high avidity for detecting a naturally presented tumor-associated antigen on osteosarcoma cells.Cancer Sci. 2019 Jan;110(1):40-51. doi: 10.1111/cas.13854. Epub 2018 Dec 1. Cancer Sci. 2019. PMID: 30375705 Free PMC article.
-
HLA-A*0201-restricted CTL epitope of a novel osteosarcoma antigen, papillomavirus binding factor.J Transl Med. 2009 Jun 12;7:44. doi: 10.1186/1479-5876-7-44. J Transl Med. 2009. PMID: 19523231 Free PMC article.
-
Prognostic impact and immunogenicity of a novel osteosarcoma antigen, papillomavirus binding factor, in patients with osteosarcoma.Cancer Sci. 2008 Feb;99(2):368-75. doi: 10.1111/j.1349-7006.2008.00695.x. Cancer Sci. 2008. PMID: 18271936 Free PMC article.
-
Advances on immunotherapy for osteosarcoma.Mol Cancer. 2024 Sep 9;23(1):192. doi: 10.1186/s12943-024-02105-9. Mol Cancer. 2024. PMID: 39245737 Free PMC article. Review.
-
Genetically modified T-cell therapy for osteosarcoma.Adv Exp Med Biol. 2014;804:323-40. doi: 10.1007/978-3-319-04843-7_18. Adv Exp Med Biol. 2014. PMID: 24924183 Free PMC article. Review.
Cited by
-
Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment.Bone Res. 2023 Feb 27;11(1):11. doi: 10.1038/s41413-023-00246-z. Bone Res. 2023. PMID: 36849442 Free PMC article. Review.
-
T-Cell-Based Immunotherapy for Osteosarcoma: Challenges and Opportunities.Front Immunol. 2016 Sep 14;7:353. doi: 10.3389/fimmu.2016.00353. eCollection 2016. Front Immunol. 2016. PMID: 27683579 Free PMC article. Review.
-
Development of a T-cell receptor multimer with high avidity for detecting a naturally presented tumor-associated antigen on osteosarcoma cells.Cancer Sci. 2019 Jan;110(1):40-51. doi: 10.1111/cas.13854. Epub 2018 Dec 1. Cancer Sci. 2019. PMID: 30375705 Free PMC article.
-
Frequent expression of human leukocyte antigen class I and the status of intratumoral immune cells in alveolar soft part sarcoma.Oncol Lett. 2017 Apr;13(4):2169-2176. doi: 10.3892/ol.2017.5696. Epub 2017 Feb 8. Oncol Lett. 2017. PMID: 28454377 Free PMC article.
-
Metabolism and senescence in the immune microenvironment of osteosarcoma: focus on new therapeutic strategies.Front Endocrinol (Lausanne). 2023 Jul 11;14:1217669. doi: 10.3389/fendo.2023.1217669. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37497349 Free PMC article. Review.
References
-
- Ferrari S., Palmerini E. (2007) Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr. Opin. Oncol. 19, 341–346 - PubMed
-
- The Japanese Orthopaedic Association (JOA) Musculoskeletal Tumor Committee (2000) General Rules for Clinical and Pathological Studies on Malignant Bone Tumors, pp. 52–61, 3rd Ed., Kanabara, Tokyo
-
- Southam C. M., Marcove R. C., Levin A. G., Buchsbaum H. J., Miké V. (1972) Proceedings: clinical trial of autogenous tumor vaccine for treatment of osteogenic sarcoma. Proc. Natl. Cancer Conf. 7, 91–100 - PubMed
-
- Campbell C. J., Cohen J., Enneking W. F. (1975) Editorial: new therapies for osteogenic sarcoma. J. Bone Joint Surg. Am. 57, 143–144 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

