A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis
- PMID: 24962607
- PMCID: PMC4332939
- DOI: 10.1093/schbul/sbu094
A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis
Abstract
Social-cognitive deficits contribute to poor functional outcomes in early psychosis; however, no effective pharmacological treatments exist for these problems. This study was the first to investigate the efficacy of an extended treatment of oxytocin nasal spray combined with social cognition training (SCT) to improve social cognition, clinical symptoms, and social functioning in early psychosis. In a double-blind, randomized, placebo-controlled, between-subjects trial, 52 individuals (aged 16-35 years) diagnosed with an early psychosis schizophrenia-spectrum illness were recruited. Participants received oxytocin (24 International Units) or placebo nasal spray twice-daily for 6 weeks, combined with group SCT (2 × 1 hour weekly sessions for 6 weeks). An additional dose of oxytocin was administered before each weekly session. Assessments were conducted at baseline, post-treatment, and at 3-month follow-up. Primary outcomes included the Reading the Mind in the Eyes Test, the Scale for the Assessment of Positive and Negative Symptoms, and the Social Functioning Scale. Secondary outcomes included self-report and behavioral assessments of social cognition, symptom severity, and social functioning. Results showed that on all primary and secondary outcomes, there was no benefit of oxytocin nasal spray treatment in comparison to placebo. Exploratory post hoc analysis suggested that increased use of nasal spray was, however, associated with reductions in negative symptoms in the oxytocin condition only. This study represents the first evaluation of oxytocin treatment for early psychosis. Although results suggest no benefit of oxytocin treatment, results also highlight an urgent need to consider nasal spray delivery and dose-related variables for future clinical trials.
Keywords: emotion recognition; neuropeptides; schizophrenia; social behavior.
© The Author 2014. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
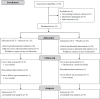


References
-
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. - PubMed
-
- Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. - PubMed
-
- Pantelis C, Yücel M, Bora E, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: the search for a moving target. Neuropsychol Rev. 2009;19:385–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

