TGF-β1 Suppresses Plasmin and MMP Activity in Flexor Tendon Cells via PAI-1: Implications for Scarless Flexor Tendon Repair
- PMID: 24962629
- PMCID: PMC4276543
- DOI: 10.1002/jcp.24707
TGF-β1 Suppresses Plasmin and MMP Activity in Flexor Tendon Cells via PAI-1: Implications for Scarless Flexor Tendon Repair
Abstract
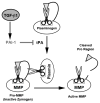
Flexor tendon injuries caused by deep lacerations to the hands are a challenging problem as they often result in debilitating adhesions that prevent the movement of the afflicted fingers. Evidence exists that tendon adhesions as well as scarring throughout the body are largely precipitated by the pleiotropic growth factor, Transforming Growth Factor Beta 1(TGF-β1), but the effects of TGF-β1 are poorly understood in tendon healing. Using an in vitro model of tendon healing, we previously found that TGF-β1 causes gene expression changes in tenocytes that are consistent with scar tissue and adhesion formation, including upregulation of the anti-fibrinolytic protein, PAI-1. Therefore, we hypothesized that TGF-β1 contributes to scarring and adhesions by reducing the activity of proteases responsible for ECM degradation and remodeling, such as plasmin and MMPs, via upregulation of PAI-1. To test our hypothesis, we examined the effects of TGF-β1 on the protease activity of tendon cells. We found that flexor tendon tenocytes treated with TGF-β1 had significantly reduced levels of active MMP-2 and plasmin. Interestingly, the effects of TGF-β1 on protease activity were completely abolished in tendon cells from homozygous plasminogen activator inhibitor 1 (PAI-1) knockout (KO) mice, which are unable to express PAI-1. Our findings support the hypothesis that TGF-β1 induces PAI-1, which suppresses plasmin and plasmin-mediated MMP activity, and provide evidence that PAI-1 may be a novel therapeutic target for preventing adhesions and promoting a scarless, regenerative repair of flexor tendon injuries.
© 2014 Wiley Periodicals, Inc.
Figures





Similar articles
-
Gene expression analysis of the pleiotropic effects of TGF-β1 in an in vitro model of flexor tendon healing.PLoS One. 2012;7(12):e51411. doi: 10.1371/journal.pone.0051411. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251524 Free PMC article.
-
Serpine1 Knockdown Enhances MMP Activity after Flexor Tendon Injury in Mice: Implications for Adhesions Therapy.Sci Rep. 2018 Apr 11;8(1):5810. doi: 10.1038/s41598-018-24144-1. Sci Rep. 2018. PMID: 29643421 Free PMC article.
-
In vitro flexor tendon cell response to TGF-beta1: a gene expression study.J Hand Surg Am. 2009 Mar;34(3):495-503. doi: 10.1016/j.jhsa.2008.10.032. J Hand Surg Am. 2009. PMID: 19258148
-
The TGF-β1/p53/PAI-1 Signaling Axis in Vascular Senescence: Role of Caveolin-1.Biomolecules. 2019 Aug 3;9(8):341. doi: 10.3390/biom9080341. Biomolecules. 2019. PMID: 31382626 Free PMC article. Review.
-
Plasminogen activator inhibitor-1 and diabetic nephropathy.Nephrology (Carlton). 2005 Oct;10 Suppl:S11-3. doi: 10.1111/j.1440-1797.2005.00449.x. Nephrology (Carlton). 2005. PMID: 16174279 Review.
Cited by
-
Molecular dissection of tendon development and healing: Insights into tenogenic phenotypes and functions.J Biol Chem. 2025 Apr;301(4):108353. doi: 10.1016/j.jbc.2025.108353. Epub 2025 Feb 25. J Biol Chem. 2025. PMID: 40015639 Free PMC article. Review.
-
Cell and Biologic-Based Treatment of Flexor Tendon Injuries.Oper Tech Orthop. 2016 Sep;26(3):206-215. doi: 10.1053/j.oto.2016.06.011. Oper Tech Orthop. 2016. PMID: 28042226 Free PMC article.
-
[In vitrodifferentiation of human amniotic mesenchymal stem cells into ligament fibroblasts after induced by transforming growth factor β 1 and vascular endothelial growth factor].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017 May 15;31(5):582-593. doi: 10.7507/1002-1892.201612090. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017. PMID: 29798549 Free PMC article. Chinese.
-
Actin Polymerization Status Regulates Tendon Homeostasis through Myocardin-Related Transcription Factor-A.bioRxiv [Preprint]. 2024 Aug 26:2024.08.26.609684. doi: 10.1101/2024.08.26.609684. bioRxiv. 2024. Update in: Cytoskeleton (Hoboken). 2025 Sep;82(9):539-555. doi: 10.1002/cm.21962. PMID: 39253450 Free PMC article. Updated. Preprint.
-
Growth factor delivery strategies for rotator cuff repair and regeneration.Int J Pharm. 2018 Jun 15;544(2):358-371. doi: 10.1016/j.ijpharm.2018.01.006. Epub 2018 Jan 6. Int J Pharm. 2018. PMID: 29317260 Free PMC article. Review.
References
-
- Caulfield RH, Maleki-Tabrizi A, Patel H, Coldham F, Mee S, Nanchahal J. Comparison of zones 1 to 4 flexor tendon repairs using absorbable and unabsorbable four-strand core sutures. The Journal of Hand Surgery. 2008;33(4):412–417. European Volume. - PubMed
-
- Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–155. - PubMed
-
- Choi HR, Kondo S, Hirose K, Ishiguro N, Hasegawa Y, Iwata H. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20(5):927–933. - PubMed
-
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. The International Journal of Biochemistry & Cell Biology. 2008;40(6–7):1362–1378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

