Image reconstruction: an overview for clinicians
- PMID: 24962650
- PMCID: PMC4276738
- DOI: 10.1002/jmri.24687
Image reconstruction: an overview for clinicians
Abstract
Image reconstruction plays a critical role in the clinical use of magnetic resonance imaging (MRI). The MRI raw data is not acquired in image space and the role of the image reconstruction process is to transform the acquired raw data into images that can be interpreted clinically. This process involves multiple signal processing steps that each have an impact on the image quality. This review explains the basic terminology used for describing and quantifying image quality in terms of signal-to-noise ratio and point spread function. In this context, several commonly used image reconstruction components are discussed. The image reconstruction components covered include noise prewhitening for phased array data acquisition, interpolation needed to reconstruct square pixels, raw data filtering for reducing Gibbs ringing artifacts, Fourier transforms connecting the raw data with image space, and phased array coil combination. The treatment of phased array coils includes a general explanation of parallel imaging as a coil combination technique. The review is aimed at readers with no signal processing experience and should enable them to understand what role basic image reconstruction steps play in the formation of clinical images and how the resulting image quality is described.
Keywords: Gibbs ringing; image reconstruction; noise correlation; point spread function; raw data filtering; signal to noise ratio.
© 2014 Wiley Periodicals, Inc.
Figures

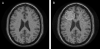

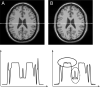


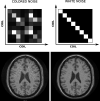





References
-
- Hinshaw WS, Lent AH. An introduction to NMR imaging: From the Bloch equation to the imaging equation. Proc. IEEE. 1983;71:338–350.
-
- Ljunggren S. A simple graphical representation of fourier-based imaging methods. J. Magn. Reson. 1983;54:338–343.
-
- Twieg DB. The k-trajectory formulation of the NMR imaging process with applications in analysis and synthesis of imaging methods. Med. Phys. 1983;10:610–21. - PubMed
-
- Hennig J. Review article K-space sampling strategies. Eur. Radiol. 1999;1031:1020–1031. - PubMed
-
- Pauly JM, Nishimura DG, Macovski A. Introduction to: A k-space analysis of small-tip-angle excitation. J. Magn. Reson. 2011;213:558–559. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

