Higher levels of interleukin IL-17 and antigen-specific IL-17 responses in pulmonary sarcoidosis patients with Löfgren's syndrome
- PMID: 24962673
- PMCID: PMC4233383
- DOI: 10.1111/cei.12403
Higher levels of interleukin IL-17 and antigen-specific IL-17 responses in pulmonary sarcoidosis patients with Löfgren's syndrome
Abstract
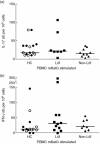
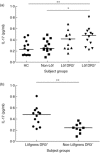
Sarcoidosis is a granulomatous disorder of unknown aetiology. The presence of Mycobacterium tuberculosis catalase-peroxidase (mKatG) in sarcoidosis tissue has been reported. T helper type 1 (Th1) responses against mKatG have previously been observed. However, little is known about interleukin (IL)-17 and Th17 responses in sarcoidosis. Here, we investigated the levels of IL-17 and frequencies of IL-17-producing cells responding to mKatG in sarcoidosis patients with different prognosis. Peripheral blood and bronchoalveolar lavage (BAL) cells were obtained from sarcoidosis patients with or without Löfgren's syndrome (often associated with spontaneous recovery), and also stratified according to human leucocyte antigen (HLA) type. Cells producing IL-17 and interferon (IFN)-γ after stimulation with mKatG were enumerated by enzyme-linked immunospot (ELISPOT). The level of IL-17 in the BAL fluid of sarcoidosis patients and healthy controls was measured by quantitative immuno-polymerase chain reaction (qIPCR). We also performed flow cytometry and immunohistochemistry for further characterization of IL-17 expression. Patients with Löfgren's syndrome had a higher frequency of IL-17-producing cells responding to mKatG in BAL fluid compared to patients without Löfgren's syndrome (P < 0·05). The HLA-DR3(+) sarcoidosis patients with Löfgren's syndrome (known to have a particularly good prognosis) also had a clearly higher level of IL-17 in BAL fluid compared to healthy controls and sarcoidosis patients without Löfgren's syndrome (P < 0·01) and (P < 0·05), respectively. No such difference between patient groups was observed with regard to IFN-γ and not with regard to either cytokine in peripheral blood. These findings suggest that IL-17-producing cells may be a useful biomarker for the prognosis of sarcoidosis and play a role in the spontaneous recovery typical of patients with Löfgren's syndrome.
Keywords: IL-17; T cells; cytokines; inflammation; sarcoidosis.
© 2014 British Society for Immunology.
Figures




Similar articles
-
Antigen-specific multifunctional T-cells in sarcoidosis patients with Lofgren's syndrome.Eur Respir J. 2012 Jul;40(1):110-21. doi: 10.1183/09031936.00166110. Epub 2011 Dec 19. Eur Respir J. 2012. PMID: 22183480
-
Th17-lineage cells in pulmonary sarcoidosis and Löfgren's syndrome: Friend or foe?J Autoimmun. 2018 Feb;87:82-96. doi: 10.1016/j.jaut.2017.12.012. Epub 2018 Jan 5. J Autoimmun. 2018. PMID: 29310925 Review.
-
No evidence of altered alveolar macrophage polarization, but reduced expression of TLR2, in bronchoalveolar lavage cells in sarcoidosis.Respir Res. 2010 Sep 2;11(1):121. doi: 10.1186/1465-9921-11-121. Respir Res. 2010. PMID: 20813038 Free PMC article.
-
Altered expression of T cell immunoglobulin-mucin (TIM) molecules in bronchoalveolar lavage CD4+ T cells in sarcoidosis.Respir Res. 2009 May 29;10(1):42. doi: 10.1186/1465-9921-10-42. Respir Res. 2009. PMID: 19480659 Free PMC article.
-
A Japanese patient with Löfgren's syndrome with an HLA-DR12 allele and review of literature on Japanese patients.Tohoku J Exp Med. 2014 Oct;234(2):137-41. doi: 10.1620/tjem.234.137. Tohoku J Exp Med. 2014. PMID: 25274017 Review.
Cited by
-
Imbalanced distribution of regulatory T cells and Th17.1 cells in the peripheral blood and BALF of sarcoidosis patients: relationship to disease activity and the fibrotic radiographic phenotype.Front Immunol. 2023 Jul 13;14:1185443. doi: 10.3389/fimmu.2023.1185443. eCollection 2023. Front Immunol. 2023. PMID: 37520566 Free PMC article.
-
Reduced expression of peroxisome proliferator-activated receptor alpha in BAL and blood T cells of non-löfgren's sarcoidosis patients.J Inflamm (Lond). 2015 Apr 9;12:28. doi: 10.1186/s12950-015-0071-6. eCollection 2015. J Inflamm (Lond). 2015. PMID: 25969669 Free PMC article.
-
The Role of Diverse Immune Cells in Sarcoidosis.Front Immunol. 2021 Nov 19;12:788502. doi: 10.3389/fimmu.2021.788502. eCollection 2021. Front Immunol. 2021. PMID: 34868074 Free PMC article. Review.
-
The Circulating Treg/Th17 Cell Ratio Is Correlated with Relapse and Treatment Response in Pulmonary Sarcoidosis Patients after Corticosteroid Withdrawal.PLoS One. 2016 Feb 4;11(2):e0148207. doi: 10.1371/journal.pone.0148207. eCollection 2016. PLoS One. 2016. PMID: 26845566 Free PMC article.
-
Emerging Molecular Targets for the Treatment of Refractory Sarcoidosis.Front Med (Lausanne). 2020 Nov 24;7:594133. doi: 10.3389/fmed.2020.594133. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33330556 Free PMC article. Review.
References
-
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2013;S0140–6736:60680–60687. - PubMed
-
- Chen ES, Moller DR. Sarcoidosis – scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–467. - PubMed
-
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. - PubMed
-
- Grunewald J, Eklund A. Lofgren's syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med. 2009;179:307–312. - PubMed
-
- Grunewald J, Janson CH, Eklund A, et al. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol. 1992;22:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

