Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season
- PMID: 24962752
- PMCID: PMC7463277
- DOI: 10.1016/j.vaccine.2014.06.052
Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season
Abstract
Background: Recent studies have suggested that vaccine-induced protection against influenza may decline within one season. We reanalyzed data from a study of influenza vaccine effectiveness to determine if time since vaccination was an independent predictor of influenza A (H3N2).
Methods: Patients with acute respiratory illness were actively recruited during the 2007-2008 season. Respiratory swabs were tested for influenza, and vaccination dates were determined by a validated immunization registry. The association between influenza RT-PCR result and vaccination interval (days) was examined using multivariable logistic regression, adjusting for calendar time, age and other confounders.
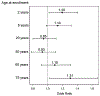
Results: There were 629 vaccinated participants, including 177 influenza A (H3N2) cases and 452 test negative controls. The mean (SD) interval from vaccination to illness onset was 101.7 (25.9) days for influenza cases and 93.0 (29.9) days for controls. There was a significant association between vaccination interval and influenza result in the main effects model. The adjusted odds ratio (aOR) for influenza was 1.12 (CI 1.01, 1.26) for every 14 day increase in the vaccination interval. Age modified the association between vaccination interval and influenza (p=0.005 for interaction). Influenza was associated with increasing vaccination interval in young children and older adults, but not in adolescents or non-elderly adults. Similar results were found when calendar week of vaccine receipt was assessed as the primary exposure variable.
Conclusions: Identification of influenza A (H3N2) was associated with increasing time since vaccination among young children and older adults during a single influenza season.
Keywords: Immunity; Influenza; Influenza vaccine; Vaccine effectiveness.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest
Dr. Belongia, Dr. Meece and Ms. Sundaram receive research support from MedImmune LLC. Other authors have no financial disclosures.
Figures

References
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36–44. - PubMed
-
- Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011;204(December (12)):1879–85. - PubMed
-
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011;30(December((12)):1081–5. - PubMed
-
- Skowronski DM, Tweed SA, Serres GD. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant. J Infect Dis 2008;197:490–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

