The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition
- PMID: 24962792
- PMCID: PMC5528583
- DOI: 10.1016/j.molonc.2014.06.001
The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition
Abstract
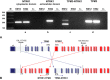
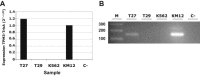
The NTRK1 gene encodes Tropomyosin-related kinase A (TRKA), the high-affinity Nerve Growth Factor Receptor. NTRK1 was originally isolated from a colorectal carcinoma (CRC) sample as component of a somatic rearrangement (TPM3-NTRK1) resulting in expression of the oncogenic chimeric protein TPM3-TRKA, but there has been no subsequent report regarding the relevance of this oncogene in CRC. The KM12 human CRC cell line expresses the chimeric TPM3-TRKA protein and is hypersensitive to TRKA kinase inhibition. We report the detailed characterization of the TPM3-NTRK1 genomic rearrangement in KM12 cells and through a cellular screening approach, the identification of NMS-P626, a novel highly potent and selective TRKA inhibitor. NMS-P626 suppressed TPM3-TRKA phosphorylation and downstream signaling in KM12 cells and showed remarkable antitumor activity in mice bearing KM12 tumors. Finally, using quantitative reverse transcriptase PCR and immunohistochemistry (IHC) we identified the TPM3-NTRK1 rearrangement in a CRC clinical sample, therefore suggesting that this chromosomal translocation is indeed a low frequency recurring event in CRC and that such patients might benefit from therapy with TRKA kinase inhibitors.
Keywords: Colorectal cancer; Kinase inhibitor; NMS-P626; TPM3-NTRK1 rearrangement; TRKA.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Adem, C. , Gisselsson, D. , Dal Cin, P. , Nascimento, A.G. , 2001. ETV6 rearrangements in patients with infantile fibrosarcomas and congenital mesoblastic nephromas by fluorescence in situ hybridization. Mod. Pathol. 14, 1246–1251. - PubMed
-
- Ardini, E. , Lombardi Borgia, A. , De Ponti, C. , Amboldi, N. , Ballinari, D. , Saccardo, M.B. , Magnaghi, P. , Pesenti, E. , Isacchi, A. , Galvani, A. , 2010. Identification and Preclinical Characterization of NMS-P626, a Potent, Selective and Orally Bioavailable TrkA Inhibitor with Anti-tumor Activity in a TrkA-dependent Colorectal Cancer. Poster presented at the 22nd AACR-NCI-EORTC Symposium on Molecular Targets and Cancer Therapeutics, Berlin (Germany) 16–19 November 2010 (Abstract)
-
- Barbacid, M. , 1995. Neurotrophic factors and their receptors. Curr. Opin. Cell Biol. 7, 148–155. - PubMed
-
- Bardelli, A. , Parsons, D.W. , Silliman, N. , Ptak, J. , Szabo, S. , Saha, S. , Markowitz, S. , Willson, J.K. , Parmigiani, G. , Kinzler, K.W. , Vogelstein, B. , Velculescu, V.E. , 2003. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 300, 949 - PubMed
-
- Bardelli, A. , Siena, S. , 2010. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 28, 1254–1261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

