Cell type-specific spatial and functional coupling between mammalian brain Kv2.1 K+ channels and ryanodine receptors
- PMID: 24962901
- PMCID: PMC4139460
- DOI: 10.1002/cne.23641
Cell type-specific spatial and functional coupling between mammalian brain Kv2.1 K+ channels and ryanodine receptors
Abstract
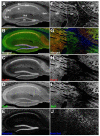
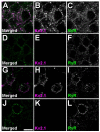
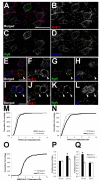
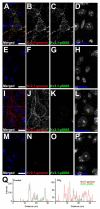
The Kv2.1 voltage-gated K+ channel is widely expressed throughout mammalian brain, where it contributes to dynamic activity-dependent regulation of intrinsic neuronal excitability. Here we show that somatic plasma membrane Kv2.1 clusters are juxtaposed to clusters of intracellular ryanodine receptor (RyR) Ca2+ -release channels in mouse brain neurons, most prominently in medium spiny neurons (MSNs) of the striatum. Electron microscopy-immunogold labeling shows that in MSNs, plasma membrane Kv2.1 clusters are adjacent to subsurface cisternae, placing Kv2.1 in close proximity to sites of RyR-mediated Ca2+ release. Immunofluorescence labeling in transgenic mice expressing green fluorescent protein in specific MSN populations reveals the most prominent juxtaposed Kv2.1:RyR clusters in indirect pathway MSNs. Kv2.1 in both direct and indirect pathway MSNs exhibits markedly lower levels of labeling with phosphospecific antibodies directed against the S453, S563, and S603 phosphorylation site compared with levels observed in neocortical neurons, although labeling for Kv2.1 phosphorylation at S563 was significantly lower in indirect pathway MSNs compared with those in the direct pathway. Finally, acute stimulation of RyRs in heterologous cells causes a rapid hyperpolarizing shift in the voltage dependence of activation of Kv2.1, typical of Ca2+ /calcineurin-dependent Kv2.1 dephosphorylation. Together, these studies reveal that striatal MSNs are distinct in their expression of clustered Kv2.1 at plasma membrane sites juxtaposed to intracellular RyRs, as well as in Kv2.1 phosphorylation state. Differences in Kv2.1 expression and phosphorylation between MSNs in direct and indirect pathways provide a cell- and circuit-specific mechanism for coupling intracellular Ca2+ release to phosphorylation-dependent regulation of Kv2.1 to dynamically impact intrinsic excitability.
Keywords: immunohistochemistry; intracellular Ca2+ release; ion channel; localization; striatum.
© 2014 Wiley Periodicals, Inc.
Figures






References
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108(1):69–81. - PubMed
-
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington’s disease transgenic mice. J Neurophysiol. 2005;93(5):2565–2574. - PubMed
-
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314(5799):615–620. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21(1):13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

