Fibroblast-like synoviocytes induce calcium mineral formation and deposition
- PMID: 24963403
- PMCID: PMC4054973
- DOI: 10.1155/2014/812678
Fibroblast-like synoviocytes induce calcium mineral formation and deposition
Abstract
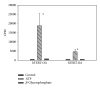
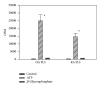
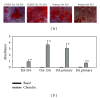
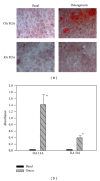
Calcium crystals are present in the synovial fluid of 65%-100% patients with osteoarthritis (OA) and 20%-39% patients with rheumatoid arthritis (RA). This study sought to investigate the role of fibroblast-like synoviocytes (FLSs) in calcium mineral formation. We found that numerous genes classified in the biomineral formation process, including bone gamma-carboxyglutamate (gla) protein/osteocalcin, runt-related transcription factor 2, ankylosis progressive homolog, and parathyroid hormone-like hormone, were differentially expressed in the OA and RA FLSs. Calcium deposits were detected in FLSs cultured in regular medium in the presence of ATP and FLSs cultured in chondrogenesis medium in the absence of ATP. More calcium minerals were deposited in the cultures of OA FLSs than in the cultures of RA FLSs. Examination of the micromass stained with nonaqueous alcoholic eosin indicated the presence of birefringent crystals. Phosphocitrate inhibited the OA FLSs-mediated calcium mineral deposition. These findings together suggest that OA FLSs are not passive bystanders but are active players in the pathological calcification process occurring in OA and that potential calcification stimuli for OA FLSs-mediated calcium deposition include ATP and certain unidentified differentiation-inducing factor(s). The OA FLSs-mediated pathological calcification process is a valid target for the development of disease-modifying drug for OA therapy.
Figures


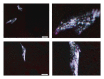
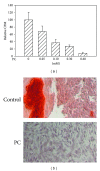
References
-
- Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. Journal of Rheumatology. 2002;29(3):570–574. - PubMed
-
- Nalbant S, Martinez JAM, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR., Jr. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis and Cartilage. 2003;11(1):50–54. - PubMed
-
- Nero P, Nogueira I, Vilar R, Pimentão JB, Branco JC. Synovial fluid crystal identification by electron microscopy. Acta Reumatologica Portuguesa. 2006;31(1):75–81. - PubMed
-
- Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis and Rheumatism. 2009;60(9):2694–2703. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

