Characterization of modified magnetite nanoparticles for albumin immobilization
- PMID: 24963410
- PMCID: PMC4054909
- DOI: 10.1155/2014/705068
Characterization of modified magnetite nanoparticles for albumin immobilization
Abstract
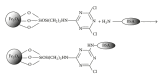
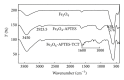
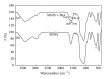
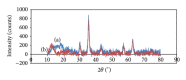
Magnetite Fe3O4 nanoparticles (NPs) were prepared by chemical coprecipitation method. Silica-coated magnetite NPs were prepared by sol-gel reaction, subsequently coated with 3-aminopropyltriethoxysilane (APTES) via silanization reaction, and then were activated with 2,4,6-trichloro-1,3,5-triazine (TCT) and covalently immobilized with bovine serum albumin (BSA). The size and structure of the particles were characterized by transmission electron microscopy (TEM), X-ray powder diffraction (XRD), and dynamic light scattering (DLS) techniques. The immobilization was confirmed by Fourier transform infrared spectroscopy (FT-IR). XRD analysis showed that the binding process has not done any phase change to Fe3O4. The immobilization time for this process was 4 h and the amount of immobilized BSA for the initial value of 1.05 mg BSA was about 120 mg/gr nanoparticles. Also, the influences of three different buffer solutions and ionic strength on covalent immobilization were evaluated.
Figures




Similar articles
-
Immobilization of albumin on aminosilane modified superparamagnetic magnetite nanoparticles and its characterization.Colloids Surf B Biointerfaces. 2009 Jun 1;71(1):154-9. doi: 10.1016/j.colsurfb.2009.01.021. Epub 2009 Feb 11. Colloids Surf B Biointerfaces. 2009. PMID: 19264459
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
The synthesis and characterization of monodispersed chitosan-coated Fe3O4 nanoparticles via a facile one-step solvothermal process for adsorption of bovine serum albumin.Nanoscale Res Lett. 2014 Jun 11;9(1):296. doi: 10.1186/1556-276X-9-296. eCollection 2014. Nanoscale Res Lett. 2014. PMID: 24994954 Free PMC article.
-
Efficient Immobilization of Porcine Pancreatic α-Amylase on Amino-Functionalized Magnetite Nanoparticles: Characterization and Stability Evaluation of the Immobilized Enzyme.Appl Biochem Biotechnol. 2016 Nov;180(5):954-968. doi: 10.1007/s12010-016-2145-1. Epub 2016 May 30. Appl Biochem Biotechnol. 2016. PMID: 27240662
-
Improving antiproliferative effect of the anticancer drug cytarabine on human promyelocytic leukemia cells by coating on Fe3O4@SiO2 nanoparticles.Colloids Surf B Biointerfaces. 2016 May 1;141:213-222. doi: 10.1016/j.colsurfb.2016.01.054. Epub 2016 Feb 1. Colloids Surf B Biointerfaces. 2016. PMID: 26852105
Cited by
-
A novel digitonin/graphene oxide/iron oxide nanocomposite: synthesis, physiochemical characterization and antioxidant activity.Discov Nano. 2024 Jan 22;19(1):15. doi: 10.1186/s11671-024-03960-7. Discov Nano. 2024. PMID: 38253925 Free PMC article.
-
The Toxicity Assessment of Iron Oxide (Fe₃O₄) Nanoparticles on Physical and Biochemical Quality of Rainbow Trout Spermatozoon.Toxics. 2018 Oct 18;6(4):62. doi: 10.3390/toxics6040062. Toxics. 2018. PMID: 30340322 Free PMC article.
-
Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities.Int J Nanomedicine. 2018 Nov 28;13:8087-8094. doi: 10.2147/IJN.S188074. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30568447 Free PMC article.
-
Controllable synthesis Fe3O4@POHABA core-shell nanostructure as high-performance recyclable bifunctional magnetic antimicrobial agent.Environ Sci Pollut Res Int. 2017 Aug;24(23):19011-19020. doi: 10.1007/s11356-017-9535-y. Epub 2017 Jun 28. Environ Sci Pollut Res Int. 2017. PMID: 28660503
-
Zinc determination in aqueous samples using energy-dispersive X-ray fluorescence spectrometry after magnetic solid-phase microextraction using Fe3O4 nanoparticles.RSC Adv. 2025 Mar 28;15(12):9569-9575. doi: 10.1039/d5ra01224d. eCollection 2025 Mar 21. RSC Adv. 2025. PMID: 40161521 Free PMC article.
References
-
- Hu H, Wang Z, Pan L. Synthesis of monodisperse Fe3O4@silica core-shell microspheres and their application for removal of heavy metal ions from water. Journal of Alloys and Compounds. 2010;492(1-2):656–661.
-
- Zhou L, Liu Z, Liu J, Huang Q. Adsorption of Hg(II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination. 2010;258(1-3):41–47.
-
- Haw CY, Mohamed F, Chia CH, et al. Hydrothermal synthesis of magnetite nanoparticles as MRI contrast agents. Ceramics International. 2010;36(4):1417–1422.
-
- Kim DK, Zhang Y, Kehr J, Klason T, Bjelke B, Muhammed M. Characterization and MRI study of surfactant-coated superparamagnetic nanoparticles administered into the rat brain. Journal of Magnetism and Magnetic Materials. 2001;225(1-2):256–261.
-
- Yoza B, Matsumoto M, Matsunaga T. DNA extraction using modified bacterial magnetic particles in the presence of amino silane compound. Journal of Biotechnology. 2002;94(3):217–224. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

