Plasmodium vivax sporozoite challenge in malaria-naïve and semi-immune Colombian volunteers
- PMID: 24963662
- PMCID: PMC4070897
- DOI: 10.1371/journal.pone.0099754
Plasmodium vivax sporozoite challenge in malaria-naïve and semi-immune Colombian volunteers
Abstract
Background: Significant progress has been recently achieved in the development of Plasmodium vivax challenge infections in humans, which are essential for vaccine and drug testing. With the goal of accelerating clinical development of malaria vaccines, the outcome of infections experimentally induced in naïve and semi-immune volunteers by infected mosquito bites was compared.
Methods: Seven malaria-naïve and nine semi-immune Colombian adults (n = 16) were subjected to the bites of 2-4 P. vivax sporozoite-infected Anopheles mosquitoes. Parasitemia levels, malaria clinical manifestations, and immune responses were assessed and compared.
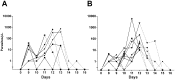
Results: All volunteers developed infections as confirmed by microscopy and RT-qPCR. No significant difference in the pre-patent period (mean 12.5 and 12.8 days for malaria-naïve and malaria-exposed, respectively) was observed but naïve volunteers developed classical malaria signs and symptoms, while semi-immune volunteers displayed minor or no symptoms at the day of diagnosis. A malaria-naïve volunteer developed a transient low submicroscopic parasitemia that cured spontaneously. Infection induced an increase in specific antibody levels in both groups.
Conclusion: Sporozoite infectious challenge was safe and reproducible in semi-immune and naïve volunteers. This model will provide information for simultaneous comparison of the protective efficacy of P. vivax vaccines in naïve and semi-immune volunteers under controlled conditions and would accelerate P. vivax vaccine development.
Trial registration: clinicaltrials.gov NCT01585077.
Conflict of interest statement
Figures

References
-
- Arevalo-Herrera M, Castellanos A, Yazdani SS, Shakri AR, Chitnis CE, et al. (2005) Immunogenicity and protective efficacy of recombinant vaccine based on the receptor-binding domain of the Plasmodium vivax Duffy binding protein in Aotus monkeys. Am J Trop Med Hyg 73: 25–31. - PubMed
-
- Bell BA, Wood JF, Bansal R, Ragab H, Cargo J 3rd, et al. (2009) Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax . Vaccine 27: 1448–1453. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

