An osmolyte mitigates the destabilizing effect of protein crowding
- PMID: 24963990
- PMCID: PMC4243990
- DOI: 10.1002/pro.2510
An osmolyte mitigates the destabilizing effect of protein crowding
Abstract
Most theories predict that macromolecular crowding stabilizes globular proteins, but recent studies show that weak attractive interactions can result in crowding-induced destabilization. Osmolytes are ubiquitous in biology and help protect cells against stress. Given that dehydration stress adds to the crowded nature of the cytoplasm, we speculated that cells might use osmolytes to overcome the destabilization caused by the increased weak interactions that accompany desiccation. We used NMR-detected amide proton exchange experiments to measure the stability of the test protein chymotrypsin inhibitor 2 under physiologically relevant crowded conditions in the presence and absence of the osmolyte glycine betaine. The osmolyte overcame the destabilizing effect of the cytosol. This result provides a physiologically relevant explanation for the accumulation of osmolytes by dehydration-stressed cells.
Keywords: amide proton exchange; macromolecular crowding; nonspecific interaction; osmolytes; protein stability.
© 2014 The Protein Society.
Figures

 in buffered 100.0 g/L protein lysate minus
in buffered 100.0 g/L protein lysate minus in buffer alone. (b)
in buffer alone. (b) in buffered 100.0 g/L protein lysate with 0.4 M glycine betaine minus
in buffered 100.0 g/L protein lysate with 0.4 M glycine betaine minus in buffered 0.4 M glycine betaine.
in buffered 0.4 M glycine betaine.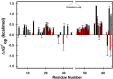

Similar articles
-
Osmotic Shock Induced Protein Destabilization in Living Cells and Its Reversal by Glycine Betaine.J Mol Biol. 2017 Apr 21;429(8):1155-1161. doi: 10.1016/j.jmb.2017.03.001. Epub 2017 Mar 3. J Mol Biol. 2017. PMID: 28263768 Free PMC article.
-
Impact of reconstituted cytosol on protein stability.Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19342-7. doi: 10.1073/pnas.1312678110. Epub 2013 Nov 11. Proc Natl Acad Sci U S A. 2013. PMID: 24218610 Free PMC article.
-
Volume exclusion and soft interaction effects on protein stability under crowded conditions.Biochemistry. 2010 Aug 24;49(33):6984-91. doi: 10.1021/bi100727y. Biochemistry. 2010. PMID: 20672856 Free PMC article.
-
Macromolecular Crowding Is More than Hard-Core Repulsions.Annu Rev Biophys. 2022 May 9;51:267-300. doi: 10.1146/annurev-biophys-091321-071829. Epub 2022 Mar 3. Annu Rev Biophys. 2022. PMID: 35239418 Review.
-
Protein and DNA destabilization by osmolytes: the other side of the coin.Life Sci. 2011 Jan 17;88(3-4):117-25. doi: 10.1016/j.lfs.2010.10.020. Epub 2010 Nov 1. Life Sci. 2011. PMID: 21047521 Review.
Cited by
-
Osmotic Shock Induced Protein Destabilization in Living Cells and Its Reversal by Glycine Betaine.J Mol Biol. 2017 Apr 21;429(8):1155-1161. doi: 10.1016/j.jmb.2017.03.001. Epub 2017 Mar 3. J Mol Biol. 2017. PMID: 28263768 Free PMC article.
-
Conditionally disordered proteins: bringing the environment back into the fold.Cell Mol Life Sci. 2017 Sep;74(17):3149-3162. doi: 10.1007/s00018-017-2558-1. Epub 2017 Jun 8. Cell Mol Life Sci. 2017. PMID: 28597298 Free PMC article. Review.
-
Crowding in Cellular Environments at an Atomistic Level from Computer Simulations.J Phys Chem B. 2017 Aug 31;121(34):8009-8025. doi: 10.1021/acs.jpcb.7b03570. Epub 2017 Jul 12. J Phys Chem B. 2017. PMID: 28666087 Free PMC article.
-
Protecting activity of desiccated enzymes.Protein Sci. 2019 May;28(5):941-951. doi: 10.1002/pro.3604. Epub 2019 Mar 30. Protein Sci. 2019. PMID: 30868674 Free PMC article.
-
Cosolutes, Crowding, and Protein Folding Kinetics.J Phys Chem B. 2017 Jul 13;121(27):6527-6537. doi: 10.1021/acs.jpcb.7b03786. Epub 2017 Jun 29. J Phys Chem B. 2017. PMID: 28605189 Free PMC article.
References
-
- Tanford C, Reynolds J. Nature's robots: a history of proteins. Oxford, UK: Oxford University Press; 2001.
-
- Creighton TE. 2010. The biophysical chemistry of nucleic acids and proteins. Eastbourne, UK: Helvetian Press.
-
- Zimmerman SB, Trach SO. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991;222:599–620. - PubMed
-
- Blattner F. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. - PubMed
-
- Neidhardt FC. Chemical composition of Escherichia coli. Vol. 1. Washington, D.C: American Society of Microbiology; 1987.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

