Identification of neuropeptide S antagonists: structure-activity relationship studies, X-ray crystallography, and in vivo evaluation
- PMID: 24964000
- PMCID: PMC4140596
- DOI: 10.1021/cn500113c
Identification of neuropeptide S antagonists: structure-activity relationship studies, X-ray crystallography, and in vivo evaluation
Abstract
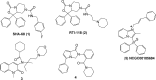
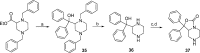
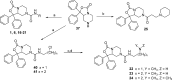
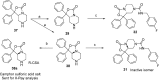
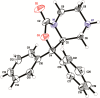
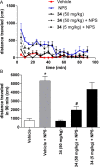
Modulation of the neuropeptide S (NPS) system has been linked to a variety of CNS disorders such as panic disorder, anxiety, sleeping disorders, asthma, obesity, PTSD, and substance abuse. In this study, a series of diphenyltetrahydro-1H-oxazolo[3,4-α]pyrazin-3(5H)-ones were synthesized and evaluated for antagonist activity at the neuropeptide S receptor. The absolute configuration was determined by chiral resolution of the key synthetic intermediate, followed by analysis of one of the individual enantiomers by X-ray crystallography. The R isomer was then converted to a biologically active compound (34) that had a Ke of 36 nM. The most potent compound displayed enhanced aqueous solubility compared with the prototypical antagonist SHA-68 and demonstrated favorable pharmacokinetic properties for behavioral assessment. In vivo analysis in mice indicated a significant blockade of NPS induced locomotor activity at an ip dose of 50 mg/kg. This suggests that analogs having improved drug-like properties will facilitate more detailed studies of the neuropeptide S receptor system.
Figures


References
-
- Sato S. Shintani Y.; Miyajima N.; Yoshimura K., Novel G-protein coupled receptor protein and DNA thereof. World Patent Application WO 2002031145 A1, 2002.
-
- Xu Y. L.; Reinscheid R. K.; Huitron-Resendiz S.; Clark S. D.; Wang Z.; Lin S. H.; Brucher F. A.; Zeng J.; Ly N. K.; Henriksen S. J.; de Lecea L.; Civelli O.; Neuropeptide S. (2004) A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43(4), 487–497. - PubMed
-
- Xu Y. L.; Gall C. M.; Jackson V. R.; Civelli O.; Reinscheid R. K. (2007) Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 500(1), 84–102. - PubMed
-
- Clark S. D.; Duangdao D. M.; Schulz S.; Zhang L.; Liu X.; Xu Y. L.; Reinscheid R. K. (2011) Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 519(10), 1867–1893. - PubMed
-
- Reinscheid R. K.; Xu Y. L. (2005) Neuropeptide S and its receptor: A newly deorphanized G protein-coupled receptor system. Neuroscientist 11(6), 532–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

