Dose responsive effects of subcutaneous pentosan polysulfate injection in mucopolysaccharidosis type VI rats and comparison to oral treatment
- PMID: 24964042
- PMCID: PMC4071040
- DOI: 10.1371/journal.pone.0100882
Dose responsive effects of subcutaneous pentosan polysulfate injection in mucopolysaccharidosis type VI rats and comparison to oral treatment
Abstract
Background: We previously demonstrated the benefits of daily, oral pentosan polysulfate (PPS) treatment in a rat model of mucopolysaccharidosis (MPS) type VI. Herein we compare these effects to once weekly, subcutaneous (s.c.) injection. The bioavailability of injected PPS is greater than oral, suggesting better delivery to difficult tissues such as bone and cartilage. Injected PPS also effectively treats osteoarthritis in animals, and has shown success in osteoarthritis patients.
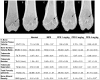
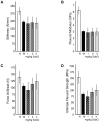
Methodology/principal findings: One-month-old MPS VI rats were given once weekly s.c. injections of PPS (1, 2 and 4 mg/kg, human equivalent dose (HED)), or daily oral PPS (4 mg/kg HED) for 6 months. Serum inflammatory markers and total glycosaminoglycans (GAGs) were measured, as were several histological, morphological and functional endpoints. Overall, weekly s.c. PPS injections led to similar or greater therapeutic effects as daily oral administration. Common findings between the two treatment approaches included reduced serum inflammatory markers, improved dentition and skull lengths, reduced tracheal deformities, and improved mobility. Enhanced effects of s.c. treatment included GAG reduction in urine and tissues, greater endurance on a rotarod, and better improvements in articular cartilage and bone in some dose groups. Optimal therapeutic effects were observed at 2 mg/kg, s.c.. No drug-related increases in liver enzymes, coagulation factor abnormalities or other adverse effects were identified following 6 months of s.c. PPS administration.
Conclusions: Once weekly s.c. administration of PPS in MPS VI rats led to equal or better therapeutic effects than daily oral administration, including a surprising reduction in urine and tissue GAGs. No adverse effects from s.c. PPS administration were observed over the 6-month study period.
Conflict of interest statement
Figures





Similar articles
-
Pentosan Polysulfate: Oral Versus Subcutaneous Injection in Mucopolysaccharidosis Type I Dogs.PLoS One. 2016 Apr 11;11(4):e0153136. doi: 10.1371/journal.pone.0153136. eCollection 2016. PLoS One. 2016. PMID: 27064989 Free PMC article.
-
Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses.PLoS One. 2013;8(1):e54459. doi: 10.1371/journal.pone.0054459. Epub 2013 Jan 24. PLoS One. 2013. PMID: 23365668 Free PMC article.
-
Open-label, single-center, clinical study evaluating the safety, tolerability and clinical effects of pentosan polysulfate sodium in subjects with mucopolysaccharidosis I.J Inherit Metab Dis. 2024 Mar;47(2):355-365. doi: 10.1002/jimd.12715. Epub 2024 Mar 11. J Inherit Metab Dis. 2024. PMID: 38467596
-
Experimental treatments for human transmissible spongiform encephalopathies: is there a role for pentosan polysulfate?Expert Opin Biol Ther. 2007 May;7(5):713-26. doi: 10.1517/14712598.7.5.713. Expert Opin Biol Ther. 2007. PMID: 17477808 Review.
-
Is there a role for pentosan polysulfate in the prevention of calcium oxalate stones?J Endourol. 2003 Dec;17(10):855-8. doi: 10.1089/089277903772036136. J Endourol. 2003. PMID: 14744348 Review.
Cited by
-
Mucopolysaccharidosis IVA: Diagnosis, Treatment, and Management.Int J Mol Sci. 2020 Feb 23;21(4):1517. doi: 10.3390/ijms21041517. Int J Mol Sci. 2020. PMID: 32102177 Free PMC article. Review.
-
Therapies for the bone in mucopolysaccharidoses.Mol Genet Metab. 2015 Feb;114(2):94-109. doi: 10.1016/j.ymgme.2014.12.001. Epub 2014 Dec 9. Mol Genet Metab. 2015. PMID: 25537451 Free PMC article. Review.
-
Pentosan Polysulfate: Oral Versus Subcutaneous Injection in Mucopolysaccharidosis Type I Dogs.PLoS One. 2016 Apr 11;11(4):e0153136. doi: 10.1371/journal.pone.0153136. eCollection 2016. PLoS One. 2016. PMID: 27064989 Free PMC article.
-
Development of Substrate Degradation Enzyme Therapy for Mucopolysaccharidosis IVA Murine Model.Int J Mol Sci. 2019 Aug 24;20(17):4139. doi: 10.3390/ijms20174139. Int J Mol Sci. 2019. PMID: 31450640 Free PMC article.
-
The effect of Tlr4 and/or C3 deficiency and of neonatal gene therapy on skeletal disease in mucopolysaccharidosis VII mice.Mol Genet Metab. 2015 Feb;114(2):209-16. doi: 10.1016/j.ymgme.2014.12.305. Epub 2014 Dec 19. Mol Genet Metab. 2015. PMID: 25559179 Free PMC article.
References
-
- Muenzer J (2011) Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 50 Suppl 5: v4–12. - PubMed
-
- Beck M, Muenzer J, Scarpa M (2010) Evaluation of disease severity in mucopolysaccharidoses. J Pediatr Rehabil Med 3: 39–46. - PubMed
-
- Lampe C, Bellettato CM, Karabul N, Scarpa M (2013) Mucopolysaccharidoses and other lysosomal storage diseases. Rheum Dis Clin North Am 39: 431–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

