Cathepsin S signals via PAR2 and generates a novel tethered ligand receptor agonist
- PMID: 24964046
- PMCID: PMC4070910
- DOI: 10.1371/journal.pone.0099702
Cathepsin S signals via PAR2 and generates a novel tethered ligand receptor agonist
Abstract
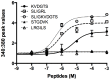
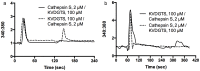
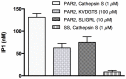
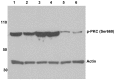
Protease-activated receptor-2 is widely expressed in mammalian epithelial, immune and neural tissues. Cleavage of PAR2 by serine proteases leads to self-activation of the receptor by the tethered ligand SLIGRL. The contribution of other classes of proteases to PAR activation has not been studied in detail. Cathepsin S is a widely expressed cysteine protease that is upregulated in inflammatory conditions. It has been suggested that cathepsin S activates PAR2. However, cathepsin S activation of PAR2 has not been demonstrated directly nor has the potential mechanism of activation been identified. We show that cathepsin S cleaves near the N-terminus of PAR2 to expose a novel tethered ligand, KVDGTS. The hexapeptide KVDGTS generates downstream signaling events specific to PAR2 but is weaker than SLIGRL. Mutation of the cathepsin S cleavage site prevents receptor activation by the protease while KVDGTS retains activity. In conclusion, the range of actions previously ascribed to cysteine cathepsins in general, and cathepsin S in particular, should be expanded to include molecular signaling. Such signaling may link together observations that had been attributed previously to PAR2 or cathepsin S individually. These interactions may contribute to inflammation.
Conflict of interest statement
Figures



Similar articles
-
Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4.J Biol Chem. 2014 Sep 26;289(39):27215-27234. doi: 10.1074/jbc.M114.599712. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118282 Free PMC article.
-
Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling.Cells. 2020 Dec 17;9(12):2704. doi: 10.3390/cells9122704. Cells. 2020. PMID: 33348659 Free PMC article.
-
Inflammatory mediators modulate thrombin and cathepsin-G signaling in human bronchial fibroblasts by inducing expression of proteinase-activated receptor-4.Am J Physiol Lung Cell Mol Physiol. 2007 Mar;292(3):L788-98. doi: 10.1152/ajplung.00226.2006. Epub 2006 Dec 1. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17142351
-
Possible role of proteases in preconditioning of brain cells to pathological conditions.Biochemistry (Mosc). 2015 Feb;80(2):163-71. doi: 10.1134/S0006297915020030. Biochemistry (Mosc). 2015. PMID: 25756531 Review.
-
Proteinase-activated receptor-2: physiological and pathophysiological roles.Curr Med Chem Cardiovasc Hematol Agents. 2003 Mar;1(1):61-72. doi: 10.2174/1568016033356715. Curr Med Chem Cardiovasc Hematol Agents. 2003. PMID: 15317291 Review.
Cited by
-
Activation of mas-related G-protein-coupled receptors by the house dust mite cysteine protease Der p1 provides a new mechanism linking allergy and inflammation.J Biol Chem. 2017 Oct 20;292(42):17399-17406. doi: 10.1074/jbc.M117.787887. Epub 2017 Aug 2. J Biol Chem. 2017. PMID: 28768771 Free PMC article.
-
Pleiotropic actions of factor Xa inhibition in cardiovascular prevention: mechanistic insights and implications for anti-thrombotic treatment.Cardiovasc Res. 2021 Jul 27;117(9):2030-2044. doi: 10.1093/cvr/cvaa263. Cardiovasc Res. 2021. PMID: 32931586 Free PMC article. Review.
-
Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4.J Biol Chem. 2014 Sep 26;289(39):27215-27234. doi: 10.1074/jbc.M114.599712. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118282 Free PMC article.
-
Potent Small Agonists of Protease Activated Receptor 2.ACS Med Chem Lett. 2015 Nov 30;7(1):105-10. doi: 10.1021/acsmedchemlett.5b00429. eCollection 2016 Jan 14. ACS Med Chem Lett. 2015. PMID: 26819675 Free PMC article.
-
Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling.Cells. 2020 Dec 17;9(12):2704. doi: 10.3390/cells9122704. Cells. 2020. PMID: 33348659 Free PMC article.
References
-
- Coughlin SR (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3: 1800–1814. - PubMed
-
- Moormann C, Artuc M, Pohl E, Varga G, Buddenkotte J, et al. (2006) Functional characterization and expression analysis of the proteinase-activated receptor-2 in human cutaneous mast cells. J Invest Dermatol 126: 746–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

