Activation of A1-adenosine receptors promotes leukocyte recruitment to the lung and attenuates acute lung injury in mice infected with influenza A/WSN/33 (H1N1) virus
- PMID: 24965449
- PMCID: PMC4136329
- DOI: 10.1128/JVI.01068-14
Activation of A1-adenosine receptors promotes leukocyte recruitment to the lung and attenuates acute lung injury in mice infected with influenza A/WSN/33 (H1N1) virus
Abstract
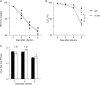
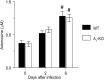
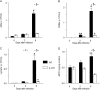
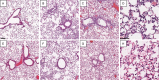
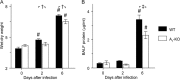
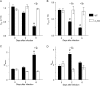
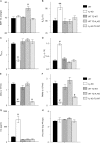
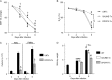
We have shown that bronchoalveolar epithelial A1-adenosine receptors (A1-AdoR) are activated in influenza A virus-infected mice. Alveolar macrophages and neutrophils also express A1-AdoRs, and we hypothesized that activation of A1-AdoRs on these cells will promote macrophage and neutrophil chemotaxis and activation and thereby play a role in the pathogenesis of influenza virus-induced acute lung injury. Wild-type (WT) C57BL/6 mice, congenic A1-AdoR knockout (A1-KO) mice, and mice that had undergone reciprocal bone marrow transfer were inoculated intranasally with 10,000 PFU/mouse influenza A/WSN/33 (H1N1) virus. Alternatively, WT mice underwent daily treatment with the A1-AdoR antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) from 1 day prior to inoculation. Infection increased bronchoalveolar lining fluid (BALF) adenosine comparably in WT and A1-KO mice. Infection of WT mice resulted in reduced carotid arterial O2 saturation (hypoxemia), lung pathology, pulmonary edema, reduced lung compliance, increased basal airway resistance, and hyperresponsiveness to methacholine. These effects were absent or significantly attenuated in A1-KO mice. Levels of BALF leukocytes, gamma interferon (IFN-γ), and interleukin 10 (IL-10) were significantly reduced in infected A1-KO mice, but levels of KC, IP-10, and MCP-1 were increased. Reciprocal bone marrow transfer resulted in WT-like lung injury severity, but BALF leukocyte levels increased only in WT and A1-KO mice with WT bone barrow. Hypoxemia, pulmonary edema, and levels of BALF alveolar macrophages, neutrophils, IFN-γ, and IL-10 were reduced in DPCPX-treated WT mice. Levels of viral replication did not differ between mouse strains or treatment groups. These findings indicate that adenosine activation of leukocyte A1-AdoRs plays a significant role in their recruitment to the infected lung and contributes to influenza pathogenesis. A1-AdoR inhibitor therapy may therefore be beneficial in patients with influenza virus-induced lung injury.
Importance: Because antiviral drugs are of limited efficacy in patients hospitalized for influenza virus-induced respiratory failure, there is an urgent need for new therapeutics that can limit the progression of lung injury and reduce influenza death rates. We show that influenza A virus infection results in increased production of the nucleoside adenosine in the mouse lung and that activation of A1-subtype adenosine receptors by adenosine contributes significantly to both recruitment of innate immune cells to the lung and development of acute lung injury following influenza virus infection. We also show that treatment with an A1-adenosine receptor antagonist reduces the severity of lung injury in influenza virus-infected mice. Our findings indicate that adenosine plays an important and previously unrecognized role in the innate immune response to influenza virus infection and suggest that drugs which can inhibit either generation of adenosine or activation of A1-adenosine receptors may be beneficial in treating influenza patients hospitalized for respiratory failure.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ, the GLa Collaborating Team MOR 2013. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med. 10:e1001558. 10.1371/journal.pmed.1001558 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

