Virus-induced tubule: a vehicle for rapid spread of virions through basal lamina from midgut epithelium in the insect vector
- PMID: 24965461
- PMCID: PMC4178856
- DOI: 10.1128/JVI.01261-14
Virus-induced tubule: a vehicle for rapid spread of virions through basal lamina from midgut epithelium in the insect vector
Abstract
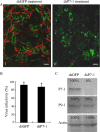
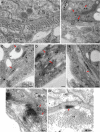
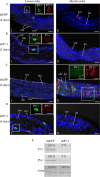
The plant reoviruses, plant rhabdoviruses, tospoviruses, and tenuiviruses are transmitted by insect vectors in a persistent propagative manner. These viruses induce the formation of viral inclusions to facilitate viral propagation in insect vectors. The intestines of insect vectors are formed by epithelial cells that lie on the noncellular basal lamina surrounded by visceral muscle tissue. Here, we demonstrate that a recently identified plant reovirus, southern rice black-streaked dwarf virus (SRBSDV), exploits virus-containing tubules composed of virus-encoded nonstructural protein P7-1 to directly cross the basal lamina from the initially infected epithelium toward visceral muscle tissues in the intestine of its vector, the white-backed planthopper (Sogatella furcifera). Furthermore, such tubules spread along visceral muscle tissues through a direct interaction of P7-1 and actin. The destruction of tubule assembly by RNA interference with synthesized double-stranded RNA targeting the P7-1 gene inhibited viral spread in the insect vector in vitro and in vivo. All these results show for the first time that a virus employs virus-induced tubule as a vehicle for viral spread from the initially infected midgut epithelium through the basal lamina, facilitating the rapid dissemination of virus from the intestine of the insect vector.
Importance: Numerous plant viruses are transmitted in a persistent manner by sap-sucking insects, including thrips, aphids, planthoppers, and leafhoppers. These viruses, ingested by the insects, establish their primary infection in the intestinal epithelium of the insect vector. Subsequently, the invading virus manages to transverse the basal lamina, a noncellular layer lining the intestine, a barrier that may theoretically hinder viral spread. The mechanism by which plant viruses cross the basal lamina is unknown. Here, we report that a plant virus has evolved to exploit virus-induced tubules to pass through the basal lamina from the initially infected midgut epithelium of the insect vector, thus revealing the previously undescribed pathway adapted by the virus for rapid dissemination of virions from the intestine of the insect vector.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Ammar ED. 1985. Internal morphology and ultrastructure of leafhoppers and planthoppers, p 121–162 In Nault LR, Rodriguez JG. (ed), Leafhoppers and planthoppers. John Wiley & Sons, Brisbane, Australia
-
- Cheung WWK, Purcell AH. 1993. Ultrastructure of the digestive system of the leafhopper Euscelidius variegatus Kirshbaum (Homoptera: Cicadellidae), with and without congenital bacterial infections. Int. J. Insect Morphol. Embryol. 22:49–61. 10.1016/0020-7322(93)90033-W - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

