Structure of the herpes simplex virus 1 genome: manipulation of nicks and gaps can abrogate infectivity and alter the cellular DNA damage response
- PMID: 24965466
- PMCID: PMC4136335
- DOI: 10.1128/JVI.01723-14
Structure of the herpes simplex virus 1 genome: manipulation of nicks and gaps can abrogate infectivity and alter the cellular DNA damage response
Abstract
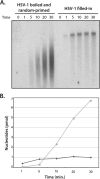
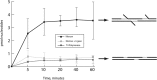
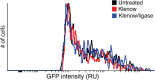
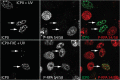
The herpes simplex virus 1 (HSV-1) virion DNA contains nicks and gaps, and in this study a novel assay for estimating the size and number of gaps in virion DNA was developed. Consistent with previous reports, we estimate that there are approximately 15 gaps per genome, and we calculate the average gap length to be approximately 30 bases. Virion DNA was isolated and treated with DNA-modifying enzymes in order to fill in the gaps and modify the ends. Interestingly, filling in gaps, blunting the ends, or adding random sequences to the 3' ends of DNA, producing 3' flaps, did not impair the infectivity of treated DNA following transfection of Vero cells. On the other hand, the formation of 5' flaps in the DNA following treatment resulted in a dramatic reduction (95 to 100%) in infectivity. Virion DNA stimulated DNA-PKcs activity in transfected cells, and DNA with 5' flaps stimulated a higher level of DNA-PKcs activity than that observed in cells transfected with untreated virion DNA. The infectivity of 5'-flapped DNA was restored in cells that do not express DNA-PKcs and in cells cotransfected with the immediate early protein ICP0, which degrades DNA-PKcs. These results are consistent with previous reports that DNA-dependent protein kinase (DNA-PK) and the nonhomologous end joining (NHEJ) repair pathway are intrinsically antiviral and that ICP0 can counteract this effect. We suggest that HSV-1 DNA with 5' flaps may induce an antiviral state due to the induction of a DNA damage response, primarily mediated by NHEJ, that renders the HSV-1 genome less efficient for lytic infection.
Importance: For productive lytic infection to occur, HSV-1 must counteract a variety of cellular intrinsic antiviral mechanisms, including the DNA damage response (DDR). DDR pathways have been associated with silencing of gene expression, cell cycle arrest, and induction of apoptosis. In addition, the fate of viral genomes is likely to play a role in whether viral genomes adopt a configuration suitable for lytic DNA replication. This study demonstrates that virion DNA activates the cellular DDR kinase, DNA-PK, and that this response is inhibitory to viral infection. Furthermore, we show that HSV-1 ubiquitin ligase, ICP0, plays an important role in counteracting the negative effects of DNA-PK activation. These findings support the notion that DNA-PK is antiviral and suggest that the fate of incoming viral DNA has important consequences for the progression of lytic infection. This study underscores the complex evolutionary relationships between HSV and its host.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

