Introductions and evolution of human-origin seasonal influenza a viruses in multinational swine populations
- PMID: 24965467
- PMCID: PMC4136342
- DOI: 10.1128/JVI.01080-14
Introductions and evolution of human-origin seasonal influenza a viruses in multinational swine populations
Abstract
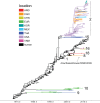
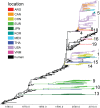
The capacity of influenza A viruses to cross species barriers presents a continual threat to human and animal health. Knowledge of the human-swine interface is particularly important for understanding how viruses with pandemic potential evolve in swine hosts. We sequenced the genomes of 141 influenza viruses collected from North American swine during 2002 to 2011 and identified a swine virus that possessed all eight genome segments of human seasonal A/H3N2 virus origin. A molecular clock analysis indicates that this virus--A/sw/Saskatchewan/02903/2009(H3N2)--has likely circulated undetected in swine for at least 7 years. For historical context, we performed a comprehensive phylogenetic analysis of an additional 1,404 whole-genome sequences from swine influenza A viruses collected globally during 1931 to 2013. Human-to-swine transmission occurred frequently over this time period, with 20 discrete introductions of human seasonal influenza A viruses showing sustained onward transmission in swine for at least 1 year since 1965. Notably, human-origin hemagglutinin (H1 and H3) and neuraminidase (particularly N2) segments were detected in swine at a much higher rate than the six internal gene segments, suggesting an association between the acquisition of swine-origin internal genes via reassortment and the adaptation of human influenza viruses to new swine hosts. Further understanding of the fitness constraints on the adaptation of human viruses to swine, and vice versa, at a genomic level is central to understanding the complex multihost ecology of influenza and the disease threats that swine and humans pose to each other.
Importance: The swine origin of the 2009 A/H1N1 pandemic virus underscored the importance of understanding how influenza A virus evolves in these animals hosts. While the importance of reassortment in generating genetically diverse influenza viruses in swine is well documented, the role of human-to-swine transmission has not been as intensively studied. Through a large-scale sequencing effort, we identified a novel influenza virus of wholly human origin that has been circulating undetected in swine for at least 7 years. In addition, we demonstrate that human-to-swine transmission has occurred frequently on a global scale over the past decades but that there is little persistence of human virus internal gene segments in swine.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier R a M, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. 10.1126/science.1176225 - DOI - PMC - PubMed
-
- Scholtissek C. 1990. Pigs as the “mixing vessel” for the creation of new pandemic influenza A viruses. Med. Princ. Pract. 2:65–71
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

