Mesenchymal stem cell contact promotes CCN1 splicing and transcription in myeloma cells
- PMID: 24965524
- PMCID: PMC4081546
- DOI: 10.1186/1478-811X-12-36
Mesenchymal stem cell contact promotes CCN1 splicing and transcription in myeloma cells
Abstract
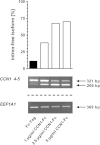
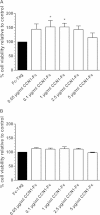
CCN family member 1 (CCN1), also known as cysteine-rich angiogenic inducer 61 (CYR61), belongs to the extracellular matrix-associated CCN protein family. The diverse functions of these proteins include regulation of cell migration, adhesion, proliferation, differentiation and survival/apoptosis, induction of angiogenesis and cellular senescence. Their functions are partly overlapping, largely non-redundant, cell-type specific, and depend on the local microenvironment. To elucidate the role of CCN1 in the crosstalk between stromal cells and myeloma cells, we performed co-culture experiments with primary mesenchymal stem cells (MSC) and the interleukin-6 (IL-6)-dependent myeloma cell line INA-6. Here we show that INA-6 cells display increased transcription and induction of splicing of intron-retaining CCN1 pre-mRNA when cultured in contact with MSC. Protein analyses confirmed that INA-6 cells co-cultured with MSC show increased levels of CCN1 protein consistent with the existence of a pre-mature stop codon in intron 1 that abolishes translation of unspliced mRNA. Addition of recombinant CCN1-Fc protein to INA-6 cells was also found to induce splicing of CCN1 pre-mRNA in a concentration-dependent manner. Only full length CCN1-Fc was able to induce mRNA splicing of all introns, whereas truncated recombinant isoforms lacking domain 4 failed to induce intron splicing. Blocking RGD-dependent integrins on INA-6 cells resulted in an inhibition of these splicing events. These findings expand knowledge on splicing of the proangiogenic, matricellular factor CCN1 in the tumor microenvironment. We propose that contact with MSC-derived CCN1 leads to splicing and enhanced transcription of CCN1 which further contributes to the translation of angiogenic factor CCN1 in myeloma cells, supporting tumor viability and myeloma bone disease.
Figures


Similar articles
-
CCN1 secreted by tonsil-derived mesenchymal stem cells promotes endothelial cell angiogenesis via integrin αv β3 and AMPK.J Cell Physiol. 2015 Jan;230(1):140-9. doi: 10.1002/jcp.24690. J Cell Physiol. 2015. PMID: 24909560
-
CYR61/CCN1 stimulates proliferation and differentiation of osteoblasts in vitro and contributes to bone remodeling in vivo in myeloma bone disease.Int J Oncol. 2017 Feb;50(2):631-639. doi: 10.3892/ijo.2016.3815. Epub 2016 Dec 22. Int J Oncol. 2017. PMID: 28035364
-
Matrix-bound Cyr61/CCN1 is required to retain the properties of the bone marrow mesenchymal stem cell niche but is depleted with aging.Matrix Biol. 2022 Aug;111:108-132. doi: 10.1016/j.matbio.2022.06.004. Epub 2022 Jun 23. Matrix Biol. 2022. PMID: 35752272 Free PMC article.
-
Biological functions and role of CCN1/Cyr61 in embryogenesis and tumorigenesis in the female reproductive system (Review).Mol Med Rep. 2018 Jan;17(1):3-10. doi: 10.3892/mmr.2017.7880. Epub 2017 Oct 26. Mol Med Rep. 2018. PMID: 29115499 Free PMC article. Review.
-
The multifunctional protein CCN1/CYR61: Bridging physiology and disease.Exp Mol Pathol. 2025 Jun;142:104969. doi: 10.1016/j.yexmp.2025.104969. Epub 2025 Apr 25. Exp Mol Pathol. 2025. PMID: 40286773 Review.
Cited by
-
The role of the CCN family of proteins in blood cancers.J Cell Commun Signal. 2016 Sep;10(3):197-205. doi: 10.1007/s12079-016-0342-x. Epub 2016 Aug 3. J Cell Commun Signal. 2016. PMID: 27485291 Free PMC article.
-
Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target?Leukemia. 2018 Jul;32(7):1500-1514. doi: 10.1038/s41375-018-0061-9. Epub 2018 Feb 22. Leukemia. 2018. PMID: 29535427 Free PMC article. Review.
-
CCN2 (Cellular Communication Network factor 2) in the bone marrow microenvironment, normal and malignant hematopoiesis.J Cell Commun Signal. 2021 Mar;15(1):25-56. doi: 10.1007/s12079-020-00602-2. Epub 2021 Jan 11. J Cell Commun Signal. 2021. PMID: 33428075 Free PMC article. Review.
-
The KISS1 Receptor as an In Vivo Microenvironment Imaging Biomarker of Multiple Myeloma Bone Disease.PLoS One. 2016 May 9;11(5):e0155087. doi: 10.1371/journal.pone.0155087. eCollection 2016. PLoS One. 2016. PMID: 27158817 Free PMC article.
-
A Negative Feedback Loop Regulates Integrin Inactivation and Promotes Neutrophil Recruitment to Inflammatory Sites.J Immunol. 2019 Sep 15;203(6):1579-1588. doi: 10.4049/jimmunol.1900443. Epub 2019 Aug 19. J Immunol. 2019. PMID: 31427445 Free PMC article.
References
-
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. - PubMed
-
- Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. - PubMed
-
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. - PubMed
-
- Schutze N, Lechner A, Groll C, Siggelkow H, Hufner M, Kohrle J, Jakob F. The human analog of murine cystein rich protein 61 [correction of 16] is a 1alpha,25-dihydroxyvitamin D3 responsive immediate early gene in human fetal osteoblasts: regulation by cytokines, growth factors, and serum. Endocrinology. 1998;139:1761–1770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

