T helper type 2-polarized invariant natural killer T cells reduce disease severity in acute intra-abdominal sepsis
- PMID: 24965554
- PMCID: PMC4233379
- DOI: 10.1111/cei.12404
T helper type 2-polarized invariant natural killer T cells reduce disease severity in acute intra-abdominal sepsis
Abstract
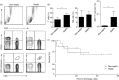
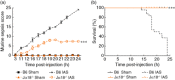
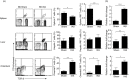
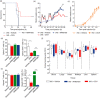
Sepsis is characterized by a severe systemic inflammatory response to infection that is associated with high morbidity and mortality despite optimal care. Invariant natural killer T (iNK T) cells are potent regulatory lymphocytes that can produce pro- and/or anti-inflammatory cytokines, thus shaping the course and nature of immune responses; however, little is known about their role in sepsis. We demonstrate here that patients with sepsis/severe sepsis have significantly elevated proportions of iNK T cells in their peripheral blood (as a percentage of their circulating T cells) compared to non-septic patients. We therefore investigated the role of iNK T cells in a mouse model of intra-abdominal sepsis (IAS). Our data show that iNK T cells are pathogenic in IAS, and that T helper type 2 (Th2) polarization of iNK T cells using the synthetic glycolipid OCH significantly reduces mortality from IAS. This reduction in mortality is associated with the systemic elevation of the anti-inflammatory cytokine interleukin (IL)-13 and reduction of several proinflammatory cytokines within the spleen, notably interleukin (IL)-17. Finally, we show that treatment of sepsis with OCH in mice is accompanied by significantly reduced apoptosis of splenic T and B lymphocytes and macrophages, but not natural killer cells. We propose that modulation of iNK T cell responses towards a Th2 phenotype may be an effective therapeutic strategy in early sepsis.
Keywords: Th2 response; acute intra-abdominal sepsis; glycolipids; invariant natural killer T cells.
© 2014 British Society for Immunology.
Figures

Similar articles
-
An alpha-galactosylceramide C20:2 N-acyl variant enhances anti-inflammatory and regulatory T cell-independent responses that prevent type 1 diabetes.Clin Exp Immunol. 2010 May;160(2):185-98. doi: 10.1111/j.1365-2249.2009.04074.x. Epub 2009 Dec 15. Clin Exp Immunol. 2010. PMID: 20015094 Free PMC article.
-
Experimental bone marrow failure in mice ameliorated by OCH via tippling the balance of released cytokines from Th1 to Th2.Immunopharmacol Immunotoxicol. 2012 Jun;34(3):491-8. doi: 10.3109/08923973.2011.633525. Epub 2011 Nov 29. Immunopharmacol Immunotoxicol. 2012. PMID: 22124468
-
Differential alterations of tissue T-cell subsets after sepsis.Immunol Lett. 2015 Nov;168(1):41-50. doi: 10.1016/j.imlet.2015.09.005. Epub 2015 Sep 8. Immunol Lett. 2015. PMID: 26362089 Free PMC article.
-
Invariant natural killer T (iNKT) cells in asthma: a novel insight into the pathogenesis of asthma and the therapeutic implication of glycolipid ligands for allergic diseases.Allergol Int. 2007 Mar;56(1):7-14. doi: 10.2332/allergolint.R-06-137. Epub 2007 Jan 29. Allergol Int. 2007. PMID: 17259804 Review.
-
The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation In Vivo.Front Immunol. 2018 Apr 23;9:716. doi: 10.3389/fimmu.2018.00716. eCollection 2018. Front Immunol. 2018. PMID: 29740428 Free PMC article. Review.
Cited by
-
CD1d- and MR1-Restricted T Cells in Sepsis.Front Immunol. 2015 Aug 12;6:401. doi: 10.3389/fimmu.2015.00401. eCollection 2015. Front Immunol. 2015. PMID: 26322041 Free PMC article. Review.
-
Single-cell transcriptomics uncovers key immune drivers of vaccine efficacy in cattle.BMC Genomics. 2025 Aug 18;26(1):750. doi: 10.1186/s12864-025-11915-0. BMC Genomics. 2025. PMID: 40826444 Free PMC article.
-
Sirtuins and Immuno-Metabolism of Sepsis.Int J Mol Sci. 2018 Sep 13;19(9):2738. doi: 10.3390/ijms19092738. Int J Mol Sci. 2018. PMID: 30216989 Free PMC article. Review.
-
Post-sepsis immunosuppression depends on NKT cell regulation of mTOR/IFN-γ in NK cells.J Clin Invest. 2020 Jun 1;130(6):3238-3252. doi: 10.1172/JCI128075. J Clin Invest. 2020. PMID: 32154791 Free PMC article.
-
Editorial: CD1- and MR1-Restricted T Cells in Antimicrobial Immunity.Front Immunol. 2015 Dec 2;6:611. doi: 10.3389/fimmu.2015.00611. eCollection 2015. Front Immunol. 2015. PMID: 26697007 Free PMC article. No abstract available.
References
-
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest. 2009;136:e28. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. - PubMed
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. - PubMed
-
- Dremsizov T, Clermont G, Kellum JA, Kalassian KG, Fine MJ, Angus DC. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

