Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes
- PMID: 24965587
- PMCID: PMC4216939
- DOI: 10.1152/ajpcell.00051.2014
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes
Abstract
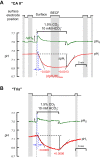
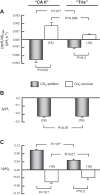
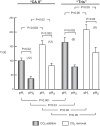
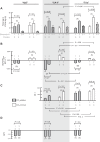
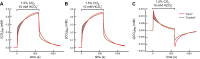
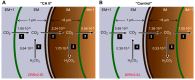
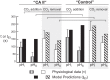
The α-carbonic anhydrases (CAs) are zinc-containing enzymes that catalyze the interconversion of CO2 and HCO3 (-). Here, we focus on human CA II (CA II), a ubiquitous cytoplasmic enzyme. In the second paper in this series, we examine CA IV at the extracellular surface. After microinjecting recombinant CA II in a Tris solution (or just Tris) into oocytes, we expose oocytes to 1.5% CO2/10 mM HCO3 (-)/pH 7.50 while using microelectrodes to monitor intracellular pH (pHi) and surface pH (pHS). CO2 influx causes the familiar sustained pHi fall as well as a transient pHS rise; CO2 efflux does the opposite. Both during CO2 addition and removal, CA II increases the magnitudes of the maximal rate of pHi change, (dpHi/dt)max, and the maximal change in pHS, ΔpHS. Preincubating oocytes with the inhibitor ethoxzolamide eliminates the effects of CA II. Compared with pHS, pHi begins to change only after a delay of ~9 s and its relaxation has a larger (i.e., slower) time constant (τpHi > τpHS ). Simultaneous measurements with two pHi electrodes, one superficial and one deep, suggest that impalement depth contributes to pHi delay and higher τpHi . Using higher CO2/HCO3 (-) levels, i.e., 5%/33 mM HCO3 (-) or 10%/66 mM HCO3 (-), increases (dpHi/dt)max and ΔpHS, though not in proportion to the increase in [CO2]. A reaction-diffusion mathematical model (described in the third paper in this series) accounts for the above general features and supports the conclusion that cytosolic CA-consuming entering CO2 or replenishing exiting CO2-increases CO2 fluxes across the cell membrane.
Keywords: electrode depth; electrophysiology; ethoxzolamide; ion-sensitive microelectrodes; mathematical modeling.
Copyright © 2014 the American Physiological Society.
Figures









Comment in
-
How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes"; "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes"; and "Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes".Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C788-90. doi: 10.1152/ajpcell.00198.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965588 No abstract available.
Similar articles
-
Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C841-58. doi: 10.1152/ajpcell.00049.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965589 Free PMC article.
-
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C814-40. doi: 10.1152/ajpcell.00050.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965590 Free PMC article.
-
How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes"; "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes"; and "Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes".Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C788-90. doi: 10.1152/ajpcell.00198.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965588 No abstract available.
-
Carotid body chemoreception: the importance of CO2-HCO3- and carbonic anhydrase. (review).Biol Res. 1993;26(3):319-29. Biol Res. 1993. PMID: 7606251 Review.
-
The renal electrogenic Na+:HCO-3 cotransporter.J Exp Biol. 1997 Jan;200(Pt 2):263-8. doi: 10.1242/jeb.200.2.263. J Exp Biol. 1997. PMID: 9050234 Review.
Cited by
-
Carbon dioxide transport across membranes.Interface Focus. 2021 Apr 6;11(2):20200090. doi: 10.1098/rsfs.2020.0090. Epub 2021 Feb 12. Interface Focus. 2021. PMID: 33633837 Free PMC article. Review.
-
Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C841-58. doi: 10.1152/ajpcell.00049.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965589 Free PMC article.
-
Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3 (-) secretion revealed by disease causing human mutation.J Physiol. 2015 Dec 15;593(24):5299-312. doi: 10.1113/JP271378. Epub 2015 Dec 7. J Physiol. 2015. PMID: 26486891 Free PMC article.
-
Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells.Oncotarget. 2017 Feb 7;8(6):10225-10237. doi: 10.18632/oncotarget.14379. Oncotarget. 2017. PMID: 28055960 Free PMC article.
-
Carbon dioxide equivalent emissions from corn silage fermentation.Front Microbiol. 2023 Jan 9;13:1092315. doi: 10.3389/fmicb.2022.1092315. eCollection 2022. Front Microbiol. 2023. PMID: 36699579 Free PMC article.
References
-
- Becker B. Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, diamox; a preliminary report. Am J Ophthalmol 37: 13–15, 1954 - PubMed
-
- Becker EL, Hodler JE, Fishman AP. Effect of carbonic anhydrase inhibitor on arterial-alveolar CO2 gradient in man. Proc Soc Exp Biol Med 84: 193–195, 1953 - PubMed
-
- Bell AL, Jr, Smith CN, Andreae E. Effects of the carbonic anhydrase inhibitor “6063” (diamox) on respiration and electrolyte metabolism of patients with respiratory acidosis. Am J Med 18: 536–546, 1955 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

